NP0139-A, NP0139-3E
UKKi035-A
General
Cell Line |
|
| hPSCreg name | UKKi035-A |
| Cite as: | UKKi035-A (RRID:CVCL_VF42) |
| Alternative name(s) |
NP0139-A, NP0139-3E
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines |
UKKi035-B (NP0139-B, NP0139-6C) Donor's gene variants: MYBPC3, MYBPC3 Donor diseases: Rare hypertrophic cardiomyopathy UKKi035-C (NP0139-C, NP0139-24D) Donor's gene variants: MYBPC3, MYBPC3 Donor diseases: Rare hypertrophic cardiomyopathy FAMRCi006-A (LMNA T3) Donor's gene variants: LMNA Donor diseases: Emery-Dreifuss Muscular Dystrophy Dilated Cardiomyopathy FAMRCi006-B (LMNA T4) Donor's gene variants: LMNA Donor diseases: Emery-Dreifuss Muscular Dystrophy Dilated Cardiomyopathy FAMRCi007-A (LMNA #23) Donor's gene variants: LMNA Donor diseases: atrioventricular block Emery-Dreifuss Muscular Dystrophy Paroxysmal atrial fibrillation FAMRCi007-B (LMNA #19) Donor's gene variants: LMNA Donor diseases: atrioventricular block Emery-Dreifuss Muscular Dystrophy Paroxysmal atrial fibrillation |
| Last update | 18th October 2019 |
| User feedback | |
Provider |
|
| Generator | Klinikum der Universität zu Köln (UKK) |
| Distributors | |
| Derivation country | Germany |
External Databases |
|
| BioSamples | SAMEA104615859 |
| EBiSC | UKKi035-A |
| Cellosaurus | CVCL_VF42 |
| Wikidata | Q54990541 |
General Information |
|
| Projects | |
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: allowed
Commercial use: allowed
|
Donor Information
General Donor Information |
|
| Sex | male |
| Age of donor (at collection) | 65-69 |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
|
Donor Relations |
|
| Other cell lines of this donor | |
External Databases (Donor) |
|
| BioSamples | SAMEA104615903 |
Ethics
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Has the donor been informed that participation will not directly influence their personal treatment? | Yes |
| Can you provide us with a copy of the Donor Information Sheet provided to the donor? | Yes |
| Do you (Depositor/Provider) hold the original Donor Consent Form? | No |
| If you do not hold the Donor Consent Form, do you know who does? | Yes |
| Contact information / weblink | Tomo Saric |
| Is there other documentation provided to the donor for consenting purposes? | No |
| Confirm that consent was obtained by a qualified professional | Yes |
| Has the donor agreed to be re-contacted? | Yes |
| Has the donor been informed about how her/his data will be protected? | Yes |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | pseudonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| Does consent expressly prevent the derivation of pluripotent stem cells? | No |
| Does consent pertain to a specific research project? | No |
| Does consent permit unforeseen future research, without further consent? | Yes |
| Does the consent permit uses of donated embryo/tissue or derived cell line intended for clinical treatment or human applications? | No |
| Does consent expressly prevent development of commercial products? | No |
| Does consent expressly prevent financial gain from any use of the donated embryo/tissue, including any product made from it? | No |
| Does consent expressly permit storage of donated embryo/tissue for an unlimited time? | Yes |
| Does consent expressly permit storage of cells derived from the donated embryo/tissue for an unlimited time? | Yes |
| Does consent prevent the DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
| Does consent prevent CELLS DERIVED FROM THE DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
Does consent permit research by | |
| an academic institution? | Yes |
| a public organisation? | Yes |
| a non-profit company? | Yes |
| a for-profit corporation? | Yes |
| Does consent expressly permit collection of genetic information? | Yes |
| Does consent expressly permit storage of genetic information? | Yes |
| Does consent prevent dissemination of genetic information? | No |
| Has the donor been informed that their donated biosample or derived cells may be tested for the presence of microbiological agents / pathogens? | Yes |
| Has the donor consented to receive information discovered during use of donated embryo/tissue that has significant health implications for the donor? | No |
| How may genetic information associated with the cell line be accessed? | Controlled Access |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Does the consent anticipate that the donor will be notified of results or outcomes of any research involving the donated samples or derived cells? | No |
| Does the consent permit the donor, upon withdrawal of consent, to stop the use of the derived cell line(s) that have already been created from donated samples? | No |
| Does the consent permit the donor, upon withdrawal of consent, to stop delivery or use of information and data about the donor? | No |
| Does consent permit access to medical records of the donor? | Yes |
| Please describe how access is provided: | Clinic in which the donor was recruted holds the medical records |
| Does consent permit access to any other source of information about the clinical treatment or health of the donor? | Yes |
| Contact data, institution, or website: | |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | Independent Ethics Committee of the Faculty of Medicin of the University of Cologne |
| Approval number | DRKS00009433 |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the PROPOSED PROJECT, involving use of donated embryo/tissue or derived cells? | Yes |
| Name of accrediting authority involved? | Committee of the Faculty of Medicin of the University of Cologne |
| Approval number | DRKS00009433 |
| Do you have obligations to third parties in regard to the use of the cell line? | No |
| Are you aware of any further constraints on the use of the donated embryo/tissue or derived cells? | No |
| Is there an MTA available for the cell line? | Yes |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | Thermo Fisher, Cyto Tune-iPS 2.0 Sendai Reprogramming Kit |
| Are you aware of any constraints on the use or distribution of the cell line from the owner or any parties identified in the query above? | No |
hIPSC Derivation
General |
|
| Source cell type |
A leukocyte with a single non-segmented nucleus in the mature form found in the circulatory pool of blood.
|
| Age of donor (at collection) | 65-69 |
| Collected in | 2016 |
| Passage number reprogrammed | 0 |
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Sendai virus |
| Genes | |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
| Files and images showing reprogramming vector expressed or silenced | |
Vector free reprogramming |
|
| Type of used vector free reprogramming factor(s) |
None
|
Other |
|
| Selection criteria for clones | morphology |
| Derived under xeno-free conditions |
Yes |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Vitronectin | ||||||
| Feeder cells |
No |
||||||
| Passage method |
Enzyme-free cell dissociation
EDTA
|
||||||
| O2 Concentration | 20 % | ||||||
| CO2 Concentration | 5 % | ||||||
| Medium |
Other medium:
Base medium: StemFlex
Main protein source: Albumine Serum concentration: 0 % Supplements
|
||||||
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | No |
||||||
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
||||||
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | No |
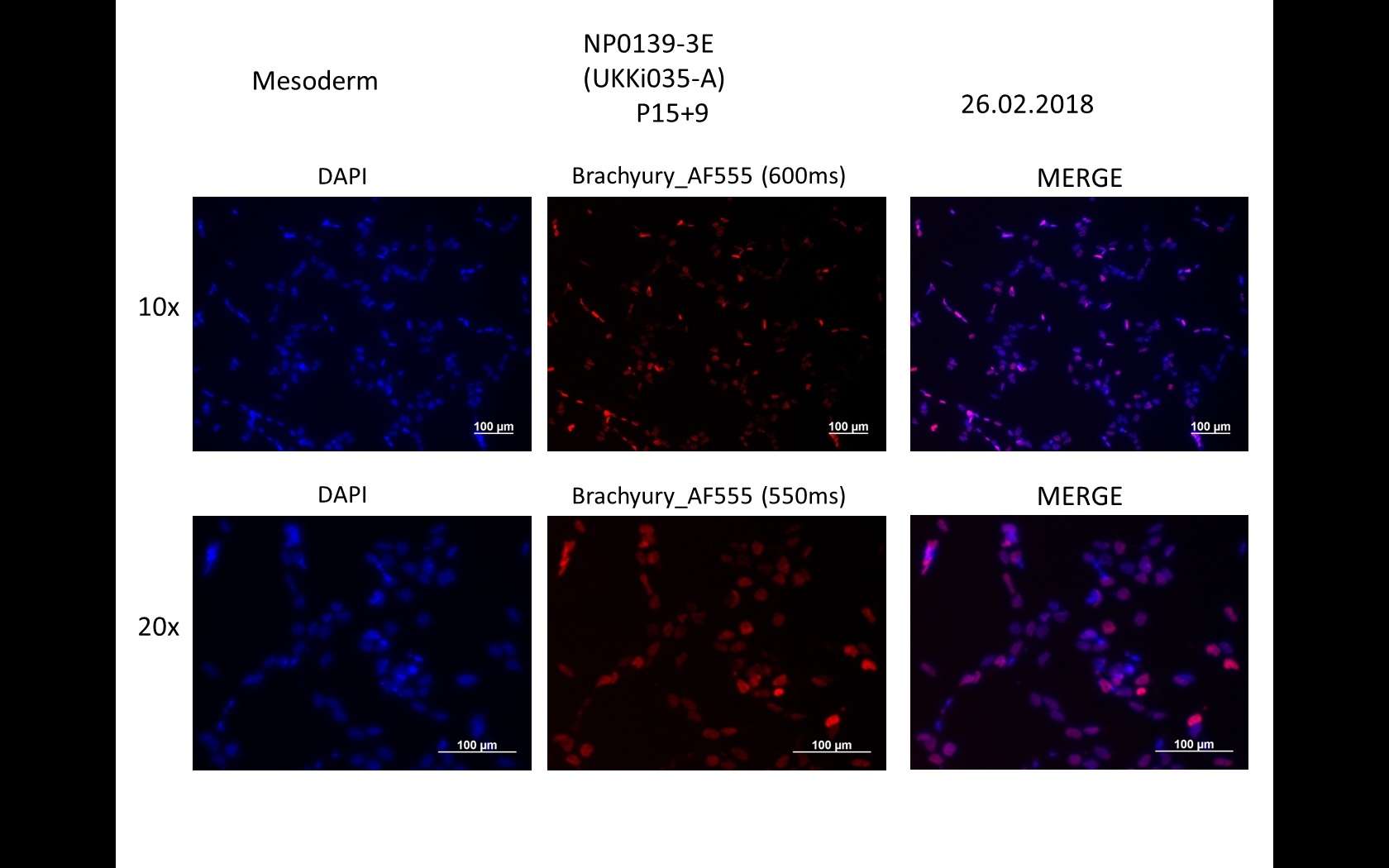
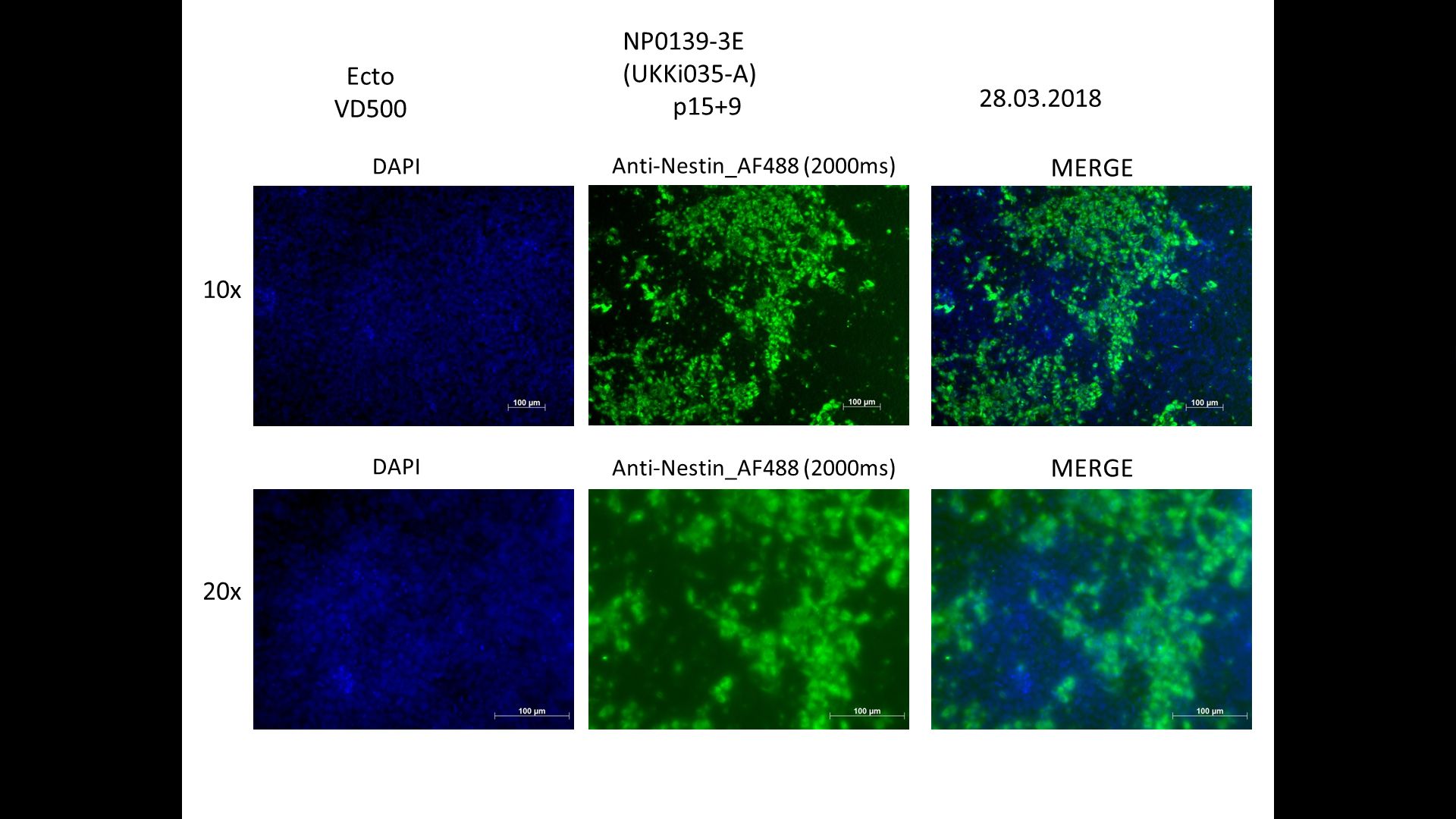
Characterisation
Analysis of Undifferentiated Cells
Differentiation Potency
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|


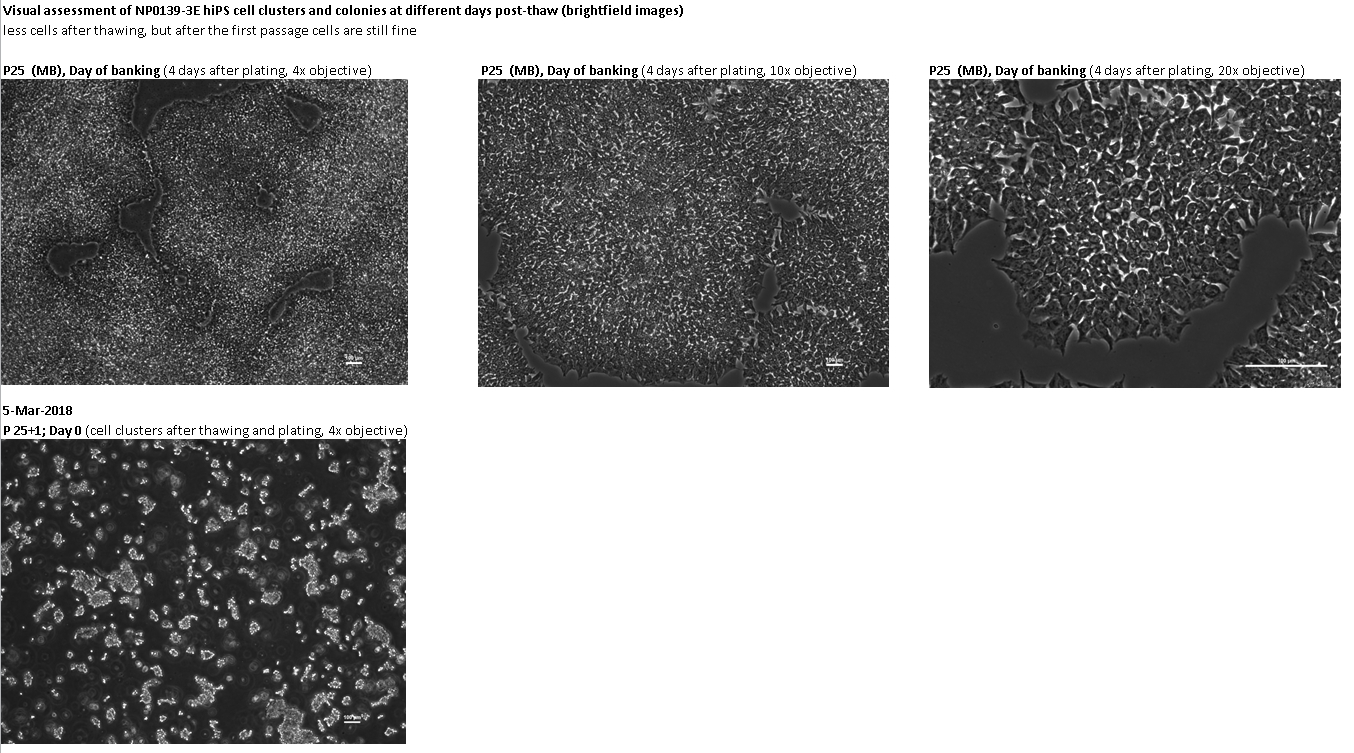
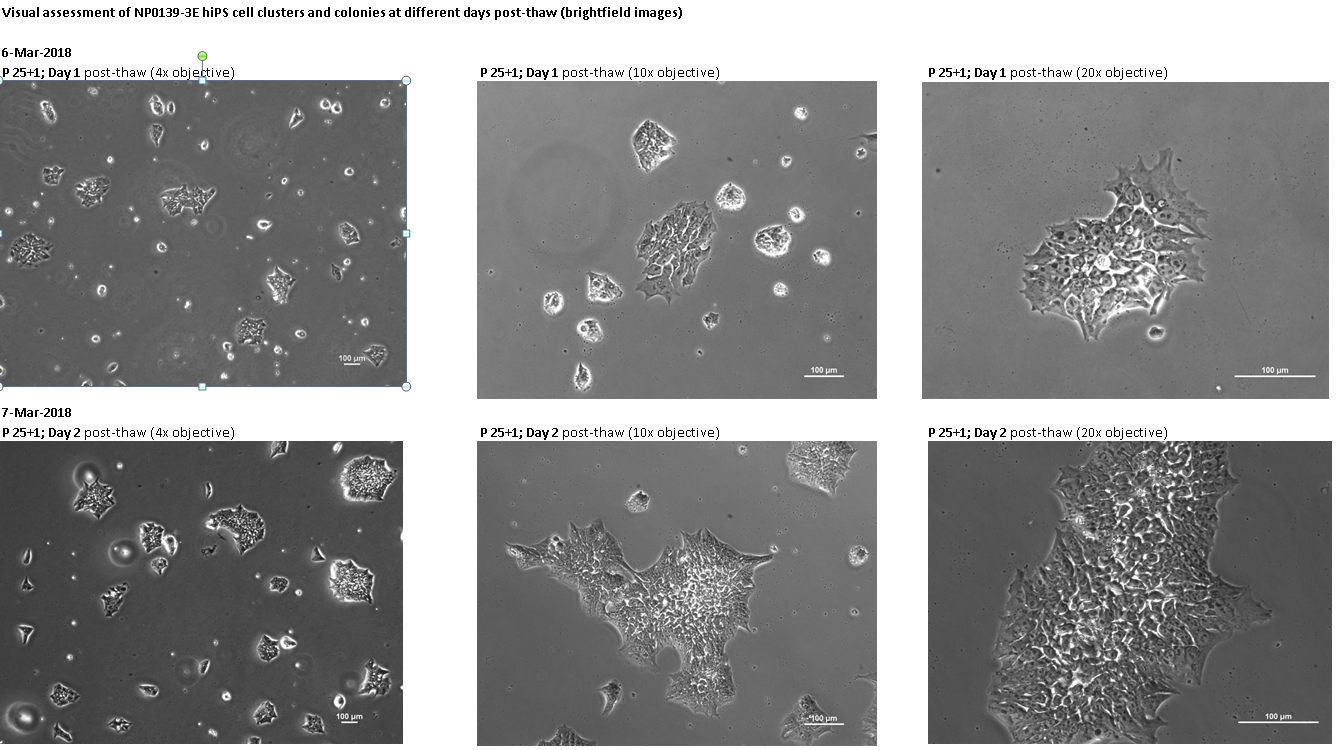
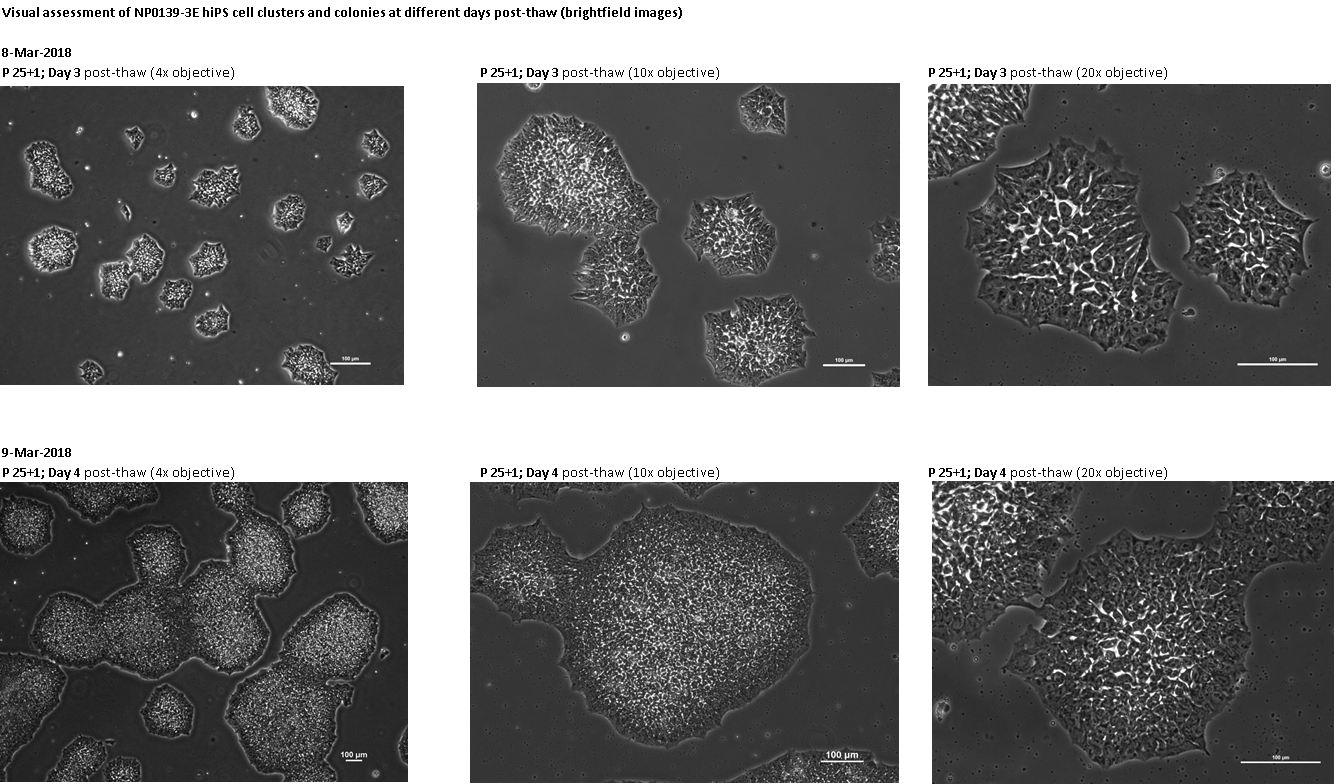
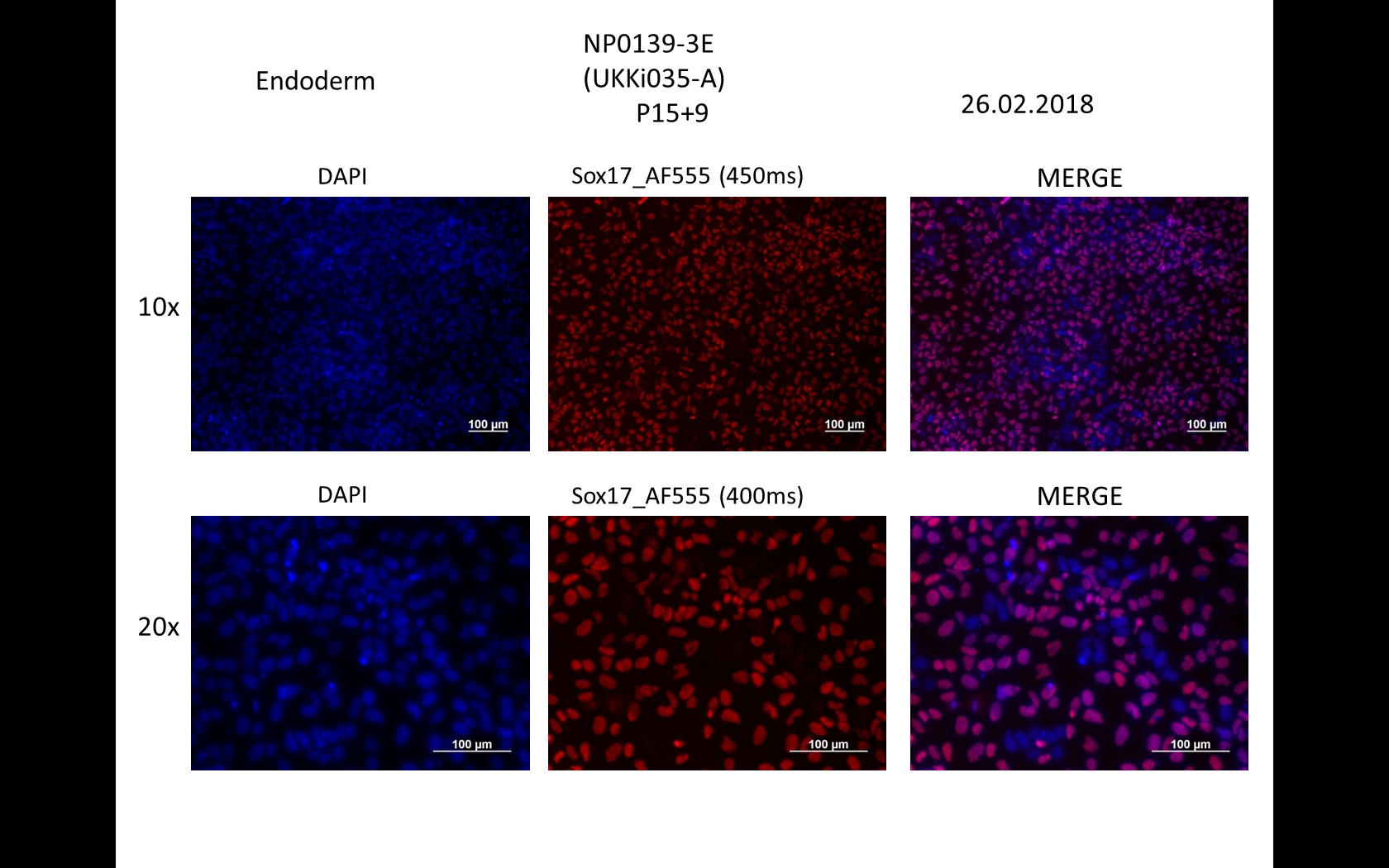
Login to share your feedback, experiences or results with the research community.