Clinical Studies Database for hPSC-derived Cell Therapies
Browse clinical studies
Register a clinical study
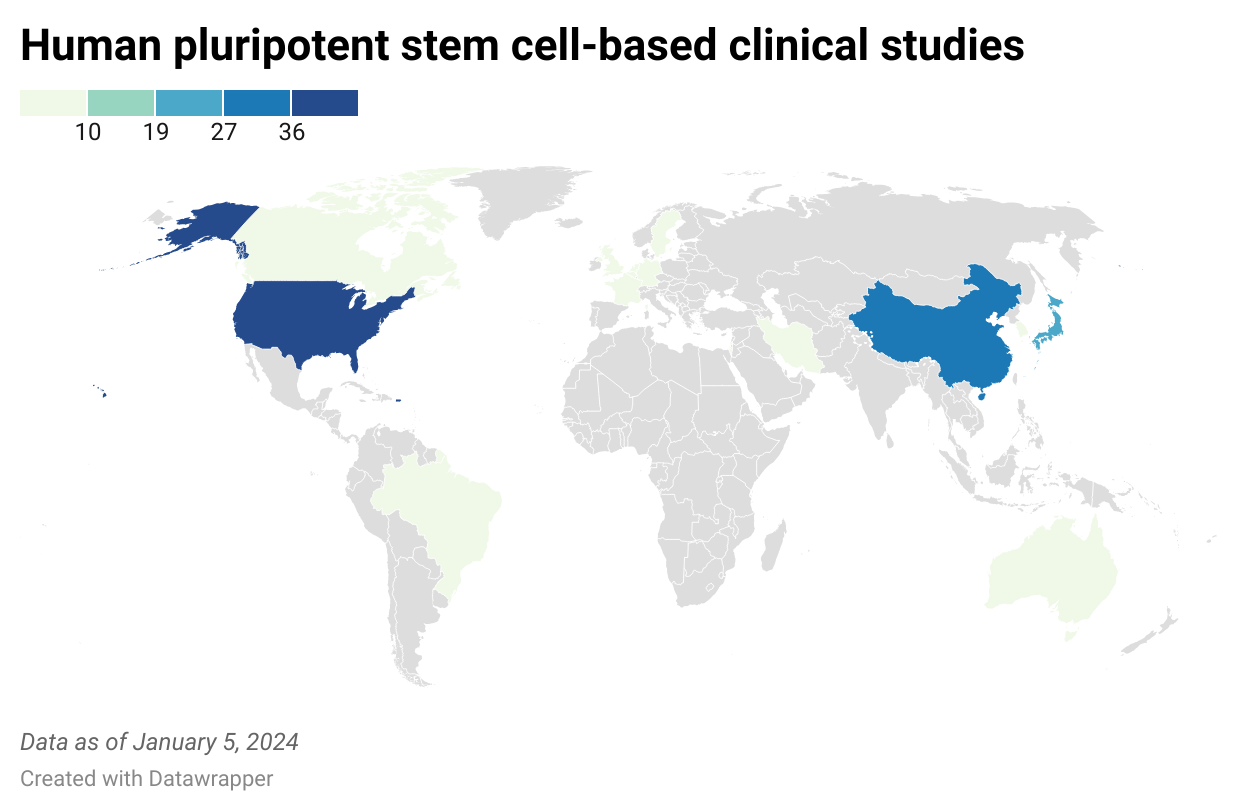
Number of clinical studies in our database: 205
About clinical studies
The clinical study database aims at providing a comprehensive overview of clinical studies that specifically use human pluripotent stem cells (hPSC) or hPSC-derived cells. These include human embryonic stem cells (hESC), human induced pluripotent stem cells (hiPSC), stem cells created by stem cell nuclear transfer (SCNT) and parthenogenetic stem cells, comprising trials worldwide where patients are actively treated with hPSC - derived cells or a product thereof.
Registration of clinical studies in hPSCreg® is (a) based on information retrieved from multiple public resources and (b) based on information provided by individual study teams.
The database also provides links to the original source of the information (i.e. clinical trial registries worldwide) and will facilitate the unique identification of trials by grouping together multiple records about the same trial.