HAD-C 102
HADe008-A
General
Cell Line |
|
| hPSCreg name | HADe008-A |
| Cite as: | HADe008-A (RRID:CVCL_B861) |
| Alternative name(s) |
HAD-C 102
|
| Cell line type | Human embryonic stem cell (hESC) |
| Similar lines |
HADe008-B (HAD-C 102 Single cell, Feeder-free (Seed Cell Bank, SCB)) |
| Last update | 14th April 2022 |
| Notes | This cell line is cGMP-grade, xeno free. It is cell-fragments on human umbilical-cord fibroblast feeders. There is a matching research-grade cell line for interested parties. Contact us if interested. |
| User feedback | |
Provider |
|
| Generator | Hadassah University Hospital (HAD) |
| Owner | Hadassah University Hospital (HAD) |
| Distributors | |
| Derivation country | Israel |
External Databases |
|
| Cellosaurus | CVCL_B861 |
| NIHhESC | NIHhESC-11-0124 |
| BioSamples | SAMEA6567198 |
General Information |
|
| Publications |
|
| * Is the cell line readily obtainable for third parties? |
Yes Research use: not allowed
Clinical use: allowed
Commercial use: allowed
Additional restrictions:
Research-use only if the ultimate goal is for clinical-applications. Please contact us if interested. There is a matching research-grade cell line available. Ethical restrictions apply. |
Donor Information
General Donor Information |
|
| Sex | male |
Phenotype and Disease related information (Donor) |
|
| Diseases | No disease was diagnosed.
|
| Disease associated phenotypes | no phenotypes |
| Family history | No known disorders |
| Is the medical history available upon request? | Brief donor summary available |
| Is clinical information available? | No medical conditions known |
Karyotyping (Donor) |
|
| Has the donor karyotype been analysed? |
No
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
No
|
Donor Relations |
|
| Other cell lines of this donor | |
External Databases (Donor) |
|
| BioSamples | SAMEA6567230 |
Ethics
| Was the embryo established purely for research purposes? | No |
| Have both parents consented to the use of the embryo for ESC derivation? | Yes |
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Has the donor been informed that participation will not directly influence their personal treatment? | Yes |
| Can you provide us with a copy of the Donor Information Sheet provided to the donor? | Yes |
| Do you (Depositor/Provider) hold the original Donor Consent Form? | Yes |
| Alternatives to consent are available? | Yes |
| Alternatives to consent | Additional info sent separa. |
| Alternative consent approval number | 0124 |
| Confirm that consent was obtained by a qualified professional | Yes |
| Has the donor agreed to be re-contacted? | No |
| Has the donor been informed about how her/his data will be protected? | Yes |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | pseudonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| * Does consent expressly prevent the derivation of pluripotent stem cells? | No |
| Does consent prevent CELLS DERIVED FROM THE DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
| How may genetic information associated with the cell line be accessed? | Controlled Access |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | IRB |
| Approval number | 33-26-07.02 |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the PROPOSED PROJECT, involving use of donated embryo/tissue or derived cells? | Yes |
| Name of accrediting authority involved? | Hadassah ESCRO Committee |
| Approval number | N/A |
hESC Derivation
| Date of derivation | 2008-12-02 |
| Embryo stage | Blastula with ICM and Trophoblast |
| Supernumerary embryos from IVF treatment? |
Yes
Separation of research and IVF treatment?
Yes |
| PGD Embryo? |
No |
| Expansion status |
6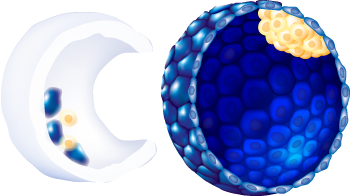
|
| ICM morphology |
A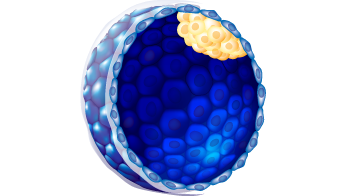
|
| Trophectoderm morphology |
a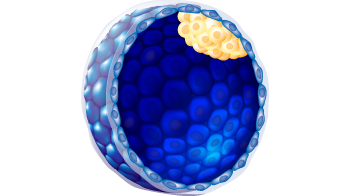
|
| Cell isolation | Laser |
| Cell seeding | Isolated ICM |
| Derived under xeno-free conditions? |
Yes |
| Derivation under GMP? |
Yes |
| Available as clinical grade? |
Yes |
Culture Conditions
| Surface coating | Gelatin |
| Feeder cells |
umbilical cord fibroblasts |
| Passage method |
Enzymatically
TrypLE
|
| O2 Concentration | 20 % |
| CO2 Concentration | 5 % |
| Medium |
Other medium:
Base medium: DMEM
Main protein source: Human Serum Serum concentration: 20 % |
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | No |
Characterisation
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| Alkaline Phosphatase |
Yes |
|
||||
| SSEA-3 |
Yes |
|
|
|||
| SSEA-4 |
Yes |
|
||||
| POU5F1 (OCT-4) |
Yes |
|
||||
| TRA 1-60 |
Yes |
|
|
|||
| TRA 1-81 |
Yes |
|
|
|||
| SSEA-1 |
No |
|
hESC Doubling Time (Potency)
Differentiation Potency
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Certificate of Analysis |
|
| Is there a certificate of analysis available? |
Yes
Passage:
|
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
XY normal
Karyotyping method:
G-Banding
|
Other Genotyping (Cell Line) |
|


Login to share your feedback, experiences or results with the research community.