SCTi003-A-3
General
Cell Line |
|
| hPSCreg name | SCTi003-A-3 |
| Cite as: | SCTi003-A-3 |
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines |
UKEi001-A-1 (CRYABhom) IDVi005-A-1 (5F-RHO-P347L-G7) IDVi005-A-2 (5F-RHO-P347L-D8) UCSDi001-A-1 (UCSD242i-LQT1-1, LQT1_2_5_iPSC_P17) UCSDi001-A-2 (LQT3_35_iPSC_P15, UCSD243i-LQT3-1) UCSDi001-A-3 (LQT3_4_28_Homo_52_iPSC_P15, UCSD244i-LQT3-2) UCSDi001-A-4 (UCSD245i-CNTL-1, T036_SNP1_Het_1_iPSC_P15_R01) UCSDi001-A-5 (UCSD246i-CNTL-2, T036_SNP1_Het_3_iPSC_P16_R01) WAe009-A-12 (H9_RB1ex3_C7) WAe009-A-13 (H9_RB1ex3_G12LS) IRFMNi003-A-3 (KO PKD1#16) NUIGi038-B-1 (CR.LQTH002Cx-A17) NUIGi038-B-2 (CR.LQTH002Cx-A21) NUIGi038-B-3 (CR.LQTH002Cx-3A15) IRFMNi003-A-4 (KO PKD1#5) NUIGi038-B-4 (CR.LQTH002Cx-3A18) IRFMNi003-A-1 (KO PKD2#17) WAe009-A-62 (KCNQ1 KO) IRFMNi003-A-2 (KO PKD2#36) IMBAi001-A-1 (HD.1 ARID1B+/- clone 3b (XX)) |
| Last update | 26th August 2025 |
| Notes | Genome-edited Human iPSC Line, SCTi003-A-3, ABCA4 Knockout (SCTi003-A-3) was generated by CRISPR/Cas9 technology, which created a loss-of-function (knockout) mutation in the ATP-binding cassette sub-family A member 4 (ABCA4) gene. ABCA4 is associated with inherited retinal disorders such as Stargardt disease, cone-rod dystrophy, and retinitis pigmentosa.
This product’s parental human induced pluripotent stem cell (hiPSC) line, Healthy Control Human hiPSC Line, Female, SCTi003-A, is a well-characterized control line derived from peripheral blood mononuclear cells (PBMCs) from a 48-year-old donor. SCTi003-A-3 has been validated by sequencing to confirm frameshift-inducing indels resulting in the complete loss of ABCA4 protein expression. Post-editing, extensive quality control procedures were undertaken in the manufacturing process for SCTi003-A-3 to ensure optimal product performance and reproducibility. SCTi003-A-3 is karyotypically stable, expresses markers of the undifferentiated state, and remains capable of directed differentiation into all three germ layers, including retinal lineage cells relevant for retinal disease research and therapeutic development. This genome-edited hiPSC line, along with its parental hiPSC line, enables precise investigation of ABCA4-linked disease mechanisms and functional genomics, as well as high-throughput screening of therapeutics targeting retinal degeneration. SCTi003-A-3 is manufactured with mTeSR™ Plus (Catalog #100-0276) and is compatible with STEMdiff™ cell culture media products, allowing for standardized high-quality maintenance and differentiation to various cell types, including retinal pigment epithelium cells. Cells were obtained using Institutional Review Board (IRB)-approved consent forms and protocols. |
| User feedback | |
Provider |
|
| Generator |
STEMCELL Technologies Inc. (SCT)
Contact:
STEMCELL Technologies Inc. (SCT) |
| Owner | STEMCELL Technologies Inc. (SCT) |
| Distributors | |
| Derivation country | United States |
External Databases |
|
| BioSamples | SAMEA119586896 |
General Information |
|
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: not allowed
Commercial use: allowed
|
| Subclone of | |
Donor Information
General Donor Information |
|
| Sex | female |
| Ethnicity |
Self-declared race/ethnicity = White Ancestry = 100% European |
Phenotype and Disease related information (Donor) |
|
| Diseases | No disease was diagnosed.
|
| Is the medical history available upon request? | No |
| Is clinical information available? | No |
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
No
|
External Databases (Donor) |
|
| BioSamples | SAMEA11371632 |
Ethics
Also have a look at the ethics information for the parental line
SCTi003-A
.
| Is there an MTA available for the cell line? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | |
| Are you aware of any constraints on the use or distribution of the cell line from the owner or any parties identified in the query above? | No |
hIPSC Derivation
General |
|
|
The source cell information can be found in the parental cell line
SCTi003-A.
|
|
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Proprietary non-integrating reprogramming technology |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
| Notes on reprogramming vector detection | Clearance confirmed at passage 21 |
Vector free reprogramming |
|
| Type of used vector free reprogramming factor(s) |
None
|
Other |
|
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
ReLeSR™
|
| O2 Concentration | 20 % |
| CO2 Concentration | 5 % |
| Medium | mTeSR™ Plus |
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | Yes |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | No |
Characterisation
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| POU5F1 (OCT-4) |
Yes |
|||||
| TRA 1-60 |
Yes |
Score:
| Marker | Present | Absent |
| mCpG | ||
| OCT4 |
Morphology pictures
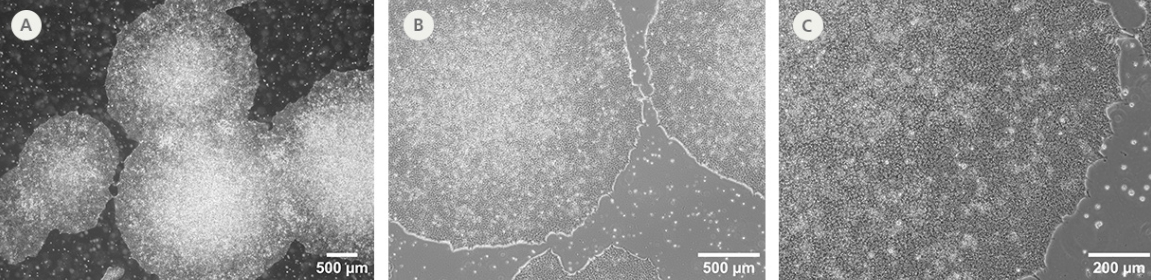
Figure 2. SCTi003-A-3 Human iPSCs Demonstrate High-Quality Morphology in Routine Culture.
Cryopreserved cells from line SCTi003-A-3 were thawed and maintained in mTeSR™ Plus (Catalog #100-1130) on Corning® Matrigel® Matrix. (A) The resulting iPSC colonies have densely packed cells and show multi-layering when ready to be passaged. (B,C) Cells retain prominent nucleoli and high nuclear-to-cytoplasmic ratios. iPSC = induced pluripotent stem cell.
Cryopreserved cells from line SCTi003-A-3 were thawed and maintained in mTeSR™ Plus (Catalog #100-1130) on Corning® Matrigel® Matrix. (A) The resulting iPSC colonies have densely packed cells and show multi-layering when ready to be passaged. (B,C) Cells retain prominent nucleoli and high nuclear-to-cytoplasmic ratios. iPSC = induced pluripotent stem cell.
Differentiation Potency
In vitro directed differentiation
Protocol or reference
In vitro directed differentiation
Protocol or reference
In vitro directed differentiation
Protocol or reference
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Certificate of Analysis |
|
| Is there a certificate of analysis available? |
Yes
Passage:
37
|
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
Exome sequencing
Whole exome sequencing data file (Catalog #500-0709) is available upon request for a fee for SCTi003-A-3 customers. Please contact iPSCrequests@stemcell.com for more information.
SNP typing array
SNP microarray data is included in each lot-specific COA. Please contact iPSCrequests@stemcell.com for more information.
Genome Sequencing
Whole genome sequencing data file (Catalog #500-0730) is available upon request for a fee for SCTi003-A-3 customers. Please contact iPSCrequests@stemcell.com for more information. |
Genetic Modification
| Disease/phenotype related modifications |
|


Login to share your feedback, experiences or results with the research community.