SCTi004-A
General
Cell Line |
|
| hPSCreg name | SCTi004-A |
| Cite as: | SCTi004-A (RRID:CVCL_E2TY) |
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines | No similar lines found. |
| Last update | 9th December 2024 |
| Notes | The Healthy Control Human iPSC Line, Male, SCTi004-A from STEMCELL Technologies (Cat no. #200-0769) was derived from peripheral blood mononuclear cells (PBMCs) from a 23-year-old donor. Extensive quality control procedures were undertaken in the iPSC manufacturing process to ensure optimal product performance and reproducibility. SCTi004-A is karyotypically stable, demonstrates trilineage differentiation potential, expresses markers of the undifferentiated state, and was reprogrammed using a non-integrating reprogramming technology. The cell line may be used as a healthy control for a multitude of pluripotent stem cell research applications including downstream differentiation to lineage-specific cell types and organoids. SCTi004-A is manufactured with mTeSR™ Plus and is fully compatible with STEMdiff™ cell culture media products, allowing for standardized high-quality maintenance and differentiation to various cell types such as cardiomyocytes, neurons, astrocytes, and microglia. |
| User feedback | |
Provider |
|
| Generator |
STEMCELL Technologies Inc. (SCT)
Contact:
STEMCELL Technologies Inc. (SCT) |
| Owner | STEMCELL Technologies Inc. (SCT) |
| Distributors | |
| Derivation country | United States |
External Databases |
|
| BioSamples | SAMEA115048409 |
| Cellosaurus | CVCL_E2TY |
General Information |
|
| Publications | |
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: not allowed
Commercial use: allowed
Additional restrictions:
For in vitro research use only. Not approved for diagnostic, therapeutic, or clinical applications. Not approved for human or veterinary use in vivo. |
Donor Information
General Donor Information |
|
| Sex | male |
| Age of donor (at collection) | 20-24 |
| Ethnicity |
Self-declared race/ethnicity = African American Ancestry = 100% African |
Phenotype and Disease related information (Donor) |
|
| Diseases | No disease was diagnosed.
|
Karyotyping (Donor) |
|
| Has the donor karyotype been analysed? |
No
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
No
|
External Databases (Donor) |
|
| BioSamples | SAMEA115048410 |
Ethics
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Has the donor been informed that participation will not directly influence their personal treatment? | Yes |
| Can you provide us with a copy of the Donor Information Sheet provided to the donor? | No |
| Do you (Depositor/Provider) hold the original Donor Consent Form? | No |
| If you do not hold the Donor Consent Form, do you know who does? | Yes |
| Is there other documentation provided to the donor for consenting purposes? | No |
| Confirm that consent was obtained by a qualified professional | Yes |
| Has the donor agreed to be re-contacted? | No |
| Has the donor been informed about how her/his data will be protected? | Yes |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | pseudonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| * Does consent expressly prevent the derivation of pluripotent stem cells? | No |
| * Does consent pertain to a specific research project? | No |
| Does consent permit unforeseen future research, without further consent? | Yes |
| Does the consent permit uses of donated embryo/tissue or derived cell line intended for clinical treatment or human applications? | No |
| Does consent expressly prevent development of commercial products? | No |
| Does consent expressly prevent financial gain from any use of the donated embryo/tissue, including any product made from it? | No |
| Does consent expressly permit storage of donated embryo/tissue for an unlimited time? | Yes |
| Does consent expressly permit storage of cells derived from the donated embryo/tissue for an unlimited time? | Yes |
| Does consent prevent the DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
| Does consent prevent CELLS DERIVED FROM THE DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
Does consent permit research by | |
| an academic institution? | Yes |
| a public organisation? | Yes |
| a non-profit company? | Yes |
| a for-profit corporation? | Yes |
| Does consent expressly permit collection of genetic information? | Yes |
| Does consent expressly permit storage of genetic information? | No |
| Does consent prevent dissemination of genetic information? | No |
| Has the donor been informed that their donated biosample or derived cells may be tested for the presence of microbiological agents / pathogens? | Yes |
| Has the donor consented to receive information discovered during use of donated embryo/tissue that has significant health implications for the donor? | No |
| How may genetic information associated with the cell line be accessed? | Controlled Access |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Does the consent anticipate that the donor will be notified of results or outcomes of any research involving the donated samples or derived cells? | No |
| Does the consent permit the donor, upon withdrawal of consent, to stop the use of the derived cell line(s) that have already been created from donated samples? | No |
| Does the consent permit the donor, upon withdrawal of consent, to stop delivery or use of information and data about the donor? | No |
| Does consent permit access to medical records of the donor? | No |
| Does consent permit access to any other source of information about the clinical treatment or health of the donor? | No |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | WCG IRB |
| Approval number | 20191112 |
| Do you have obligations to third parties in regard to the use of the cell line? | No |
| Are you aware of any further constraints on the use of the donated embryo/tissue or derived cells? | No |
| Is there an MTA available for the cell line? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | |
| Are you aware of any constraints on the use or distribution of the cell line from the owner or any parties identified in the query above? | No |
hIPSC Derivation
General |
|
| Source cell type |
A liquid tissue; its major function is to transport oxygen throughout the body. It also supplies the tissues with nutrients, removes waste products, and contains various components of the immune system defending the body against infection. Several hormones also travel in the blood.
Synonyms
|
| Source cell type (free text) | Non-T cell |
| Age of donor (at collection) | 20-24 |
| Collected in | 2023 |
| Source cell line vendor | Canventa Life Sciences |
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Proprietary non-integrating reprogramming technology |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
| Notes on reprogramming vector detection | Confirmed at passage 16 |
Vector free reprogramming |
|
Other |
|
| Selection criteria for clones | Clones were assessed for specific morphological characteristics indicative of high-quality iPSCs. These characteristics include colonies with smooth round edges, containing tightly packed cells and a high nucleus-to-cytoplasm ratio. |
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
ReLeSR™
|
| O2 Concentration | 20 % |
| CO2 Concentration | 5 % |
| Medium | mTeSR™ Plus |
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | No |
Characterisation
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| POU5F1 (OCT-4) |
Yes |
|||||
| TRA 1-60 |
Yes |
Morphology pictures
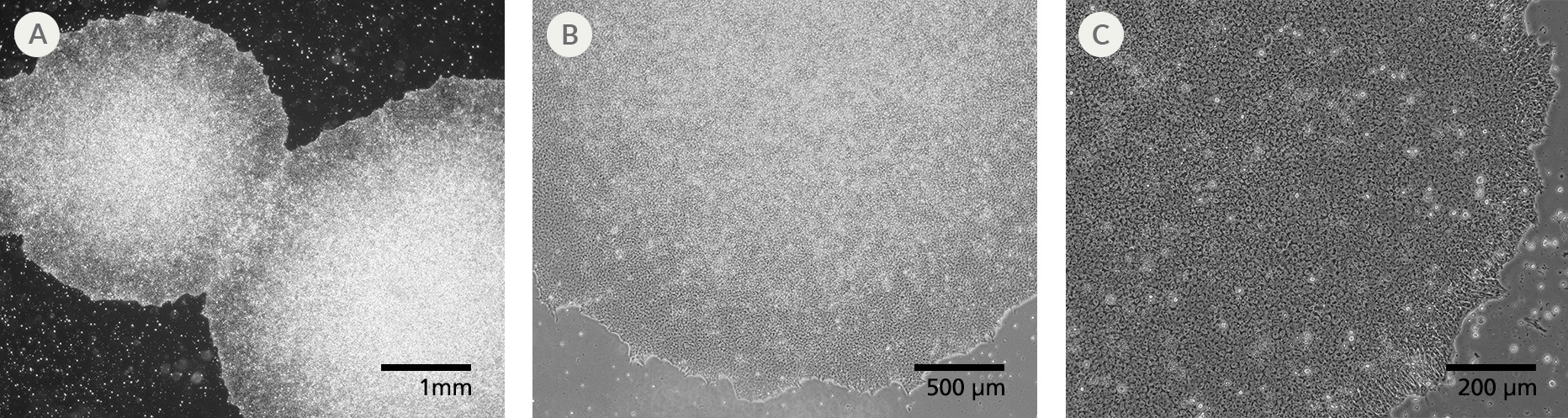
Figure 1. SCTi004-A Human Pluripotent Stem Cells Demonstrate High-Quality Morphology in Routine Culture. Cryopreserved cells from line SCTi004-A were thawed and maintained in mTeSR™ Plus on Corning® Matrigel® hESC-Qualified Matrix. (A) The resulting iPSC colonies have densely packed cells and show multi-layering when ready to be passaged. (B,C) Cells retain prominent nucleoli and high nuclear-to-cytoplasmic ratios.
Differentiation Potency
In vitro directed differentiation
Protocol or reference
In vitro directed differentiation
Protocol or reference
In vitro directed differentiation
Protocol or reference
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Certificate of Analysis |
|
| Is there a certificate of analysis available? |
Yes
Passage:
26
|
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
Exome sequencing
The whole exome sequence data file and information package (Catalog #500-0493) is available upon request for a fee for SCTi004-A customers. Please contact iPSCrequests@stemcell.com for more information.
Genome sequencing
The whole genome sequence data file and information package (Catalog #500-0494) is available upon request for a fee for SCTi004-A customers. Please contact iPSCrequests@stemcell.com for more information.
SNP typing array
Lot-specific CNV reports are included in the Certificate of Analysis (COA) for commercial SCTi004-A vials. |


Login to share your feedback, experiences or results with the research community.