MstrShef5, MShef5, MasterShef5
UOSe010-A
General
Cell Line |
|
| hPSCreg name | UOSe010-A |
| Cite as: | UOSe010-A (RRID:CVCL_BW78) |
| Alternative name(s) |
MstrShef5, MShef5, MasterShef5
|
| Cell line type | Human embryonic stem cell (hESC) |
| Similar lines |
UOSe013-A (MstrShef8, MasterShef8, MShef8) |
| Last update | 20th January 2022 |
| User feedback | |
Provider |
|
| Generator |
University of Sheffield (UOS)
Contact:
Centre for Stem Cell Biology |
| Owner | Centre for Stem Cell Biology |
| Distributors | |
| Derivation country | United Kingdom |
External Databases |
|
| BioSamples | SAMEA104627955 |
| Cellosaurus | CVCL_BW78 |
| Wikidata | Q54904051 |
General Information |
|
| Publications | |
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: allowed
Commercial use: allowed
|
Donor Information
General Donor Information |
|
| Sex | male |
Phenotype and Disease related information (Donor) |
|
| Diseases | No disease was diagnosed.
|
| Family history | No |
| Is the medical history available upon request? | No |
| Is clinical information available? | No |
Karyotyping (Donor) |
|
| Has the donor karyotype been analysed? |
No
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
No
|
Donor Relations |
|
| All cell lines of this donor's relatives |
Has brother:
|
External Databases (Donor) |
|
| BioSamples | SAMEA104627956 |
Ethics
| Was the embryo established purely for research purposes? | No |
| Have both parents consented to the use of the embryo for ESC derivation? | Yes |
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Alternatives to consent are available? | Yes |
| Alternatives to consent | |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | pseudonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| * Does consent expressly prevent the derivation of pluripotent stem cells? | No |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | National Research Ethics Service - North West London Rec-2 |
| Approval number | 05/Q0501/63 |
hESC Derivation
| Date of derivation | 2011-10-20 |
| Embryo stage | Blastula with ICM and Trophoblast |
| Supernumerary embryos from IVF treatment? |
Yes
Separation of research and IVF treatment?
Yes |
| PGD Embryo? |
No |
| Expansion status |
6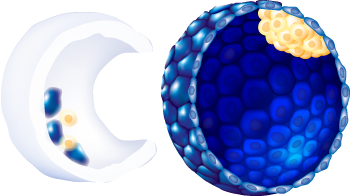
|
| ICM morphology |
C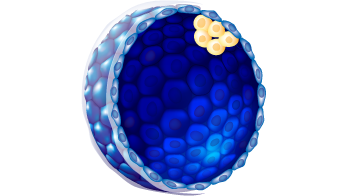
|
| Trophectoderm morphology |
g
|
| ZP removal technique | Spontaneous Hatching |
| Cell isolation | None |
| Cell seeding | Embryo |
| Derived under xeno-free conditions? |
No |
| Derivation under GMP? |
Yes |
| Available as clinical grade? |
Yes |
Culture Conditions
| Surface coating | Non coated |
| Feeder cells |
Human Foreskin Fibroblasts |
| Passage method | Mechanically |
| O2 Concentration | 20 % |
| CO2 Concentration | 5 % |
| Medium |
Other medium:
Base medium: KO-DMEM
Main protein source: Knock-out serum replacement Serum concentration: 20 % |
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | No |
Characterisation
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| SSEA-1 |
No |
|
|
|||
| SSEA-3 |
Yes |
|
|
|||
| SSEA-4 |
Yes |
|
||||
| TRA 1-60 |
Yes |
|
||||
| TRA 1-81 |
Yes |
|
|
|||
| NANOG |
Yes |
|
||||
| POU5F1 (OCT-4) |
Yes |
|
Differentiation Potency
In vitro spontaneous differentiation
| Marker | Expressed |
| alpha fetal protein |
Yes |
In vitro spontaneous differentiation
| Marker | Expressed |
| Beta Tubulin III |
Yes |
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Certificate of Analysis |
|
| Is there a certificate of analysis available? |
Yes
Passage:
|
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
46, XY
Passage number: 13
Karyotyping method:
G-Banding
|
Other Genotyping (Cell Line) |
|



Login to share your feedback, experiences or results with the research community.