1514_1 #1 CL3
The cell line is not validated yet.
CUIMCi004-A-2
General
Cell Line |
|
| hPSCreg name | CUIMCi004-A-2 |
| Cite as: | CUIMCi004-A-2 |
| Alternative name(s) |
1514_1 #1 CL3
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines |
|
| Last update | 1st November 2025 |
| User feedback | |
Provider |
|
| Generator | Columbia University Irving Medical Center (CUIMC) |
| Owner | Columbia University Irving Medical Center (CUIMC) |
| Distributors | |
| Derivation country | United States |
External Databases |
|
General Information |
|
| Publications | |
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: not allowed
Commercial use: allowed
|
| Subclone of | |
Donor Information
General Donor Information |
|
| Sex | male |
| Ethnicity | Non Hispanic White |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
No
|
Donor Relations |
|
| Other cell lines of this donor | |
External Databases (Donor) |
|
| BioSamples | SAMEA12576724 |
Ethics
Also have a look at the ethics information for the parental line
CUIMCi004-A
.
| Is there an MTA available for the cell line? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? |
hIPSC Derivation
General |
|
|
The source cell information can be found in the parental cell line
CUIMCi004-A.
|
|
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Sendai virus |
| Is reprogramming vector detectable? |
No |
| Methods used |
RT-PCR
|
Vector free reprogramming |
|
Other |
|
| Selection criteria for clones | Clones were manually picked according to their morphology, then characterized for pluripotency, stemness, and others |
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
EDTA
|
| O2 Concentration | 20 % |
| CO2 Concentration | 5 % |
| Medium | mTeSR™ Plus |
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | Yes |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | Yes |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | Yes |
Characterisation
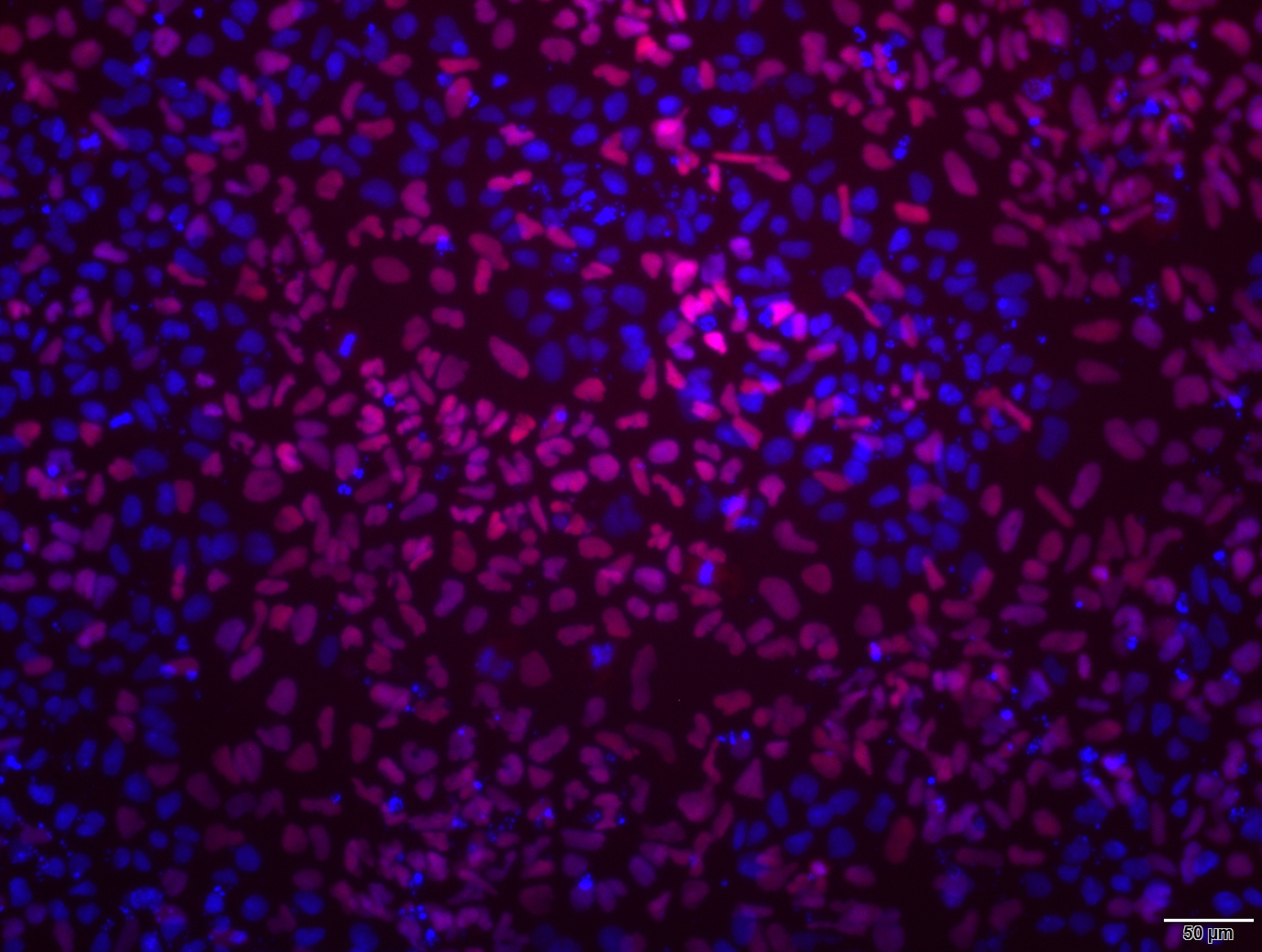
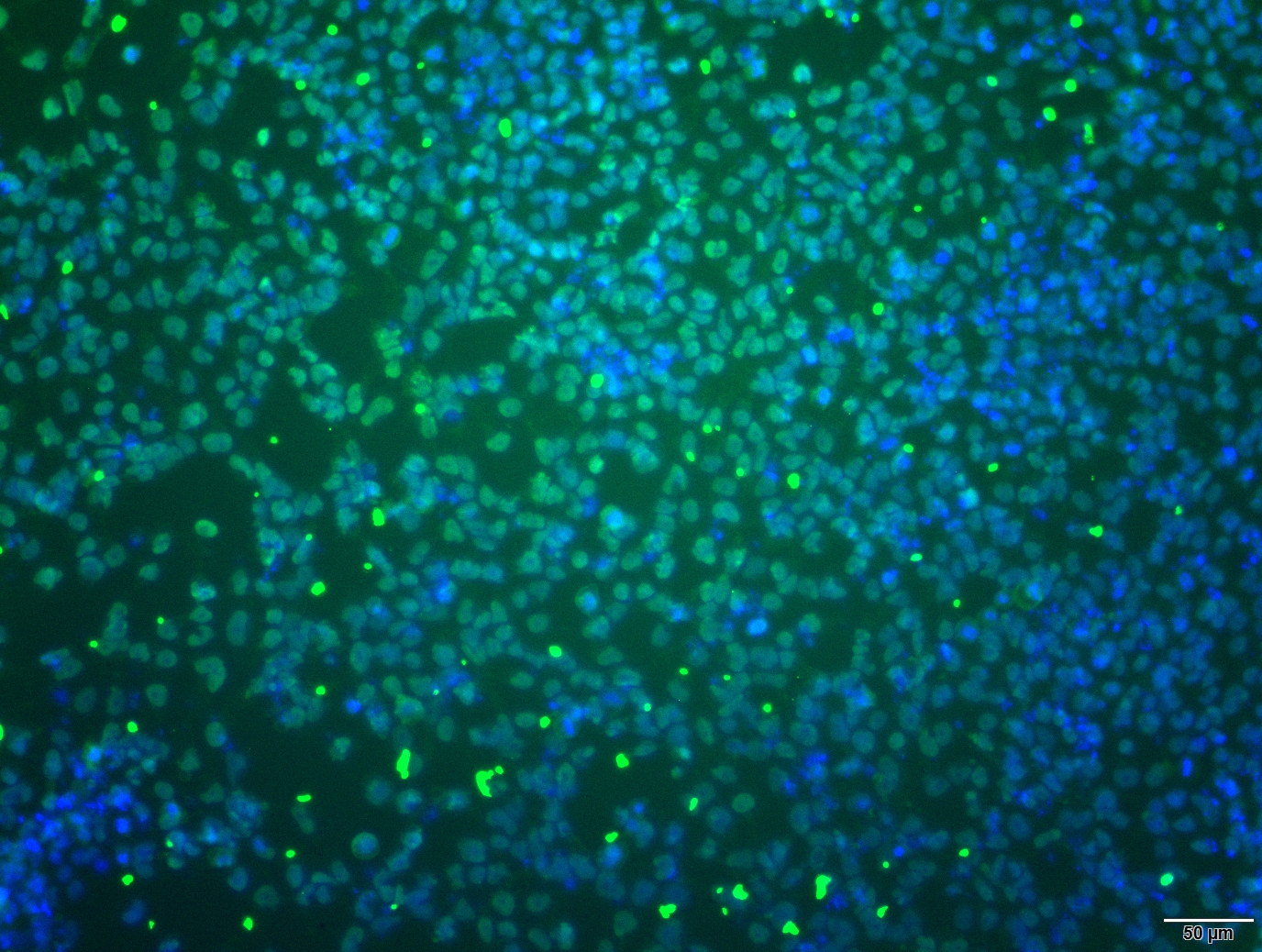
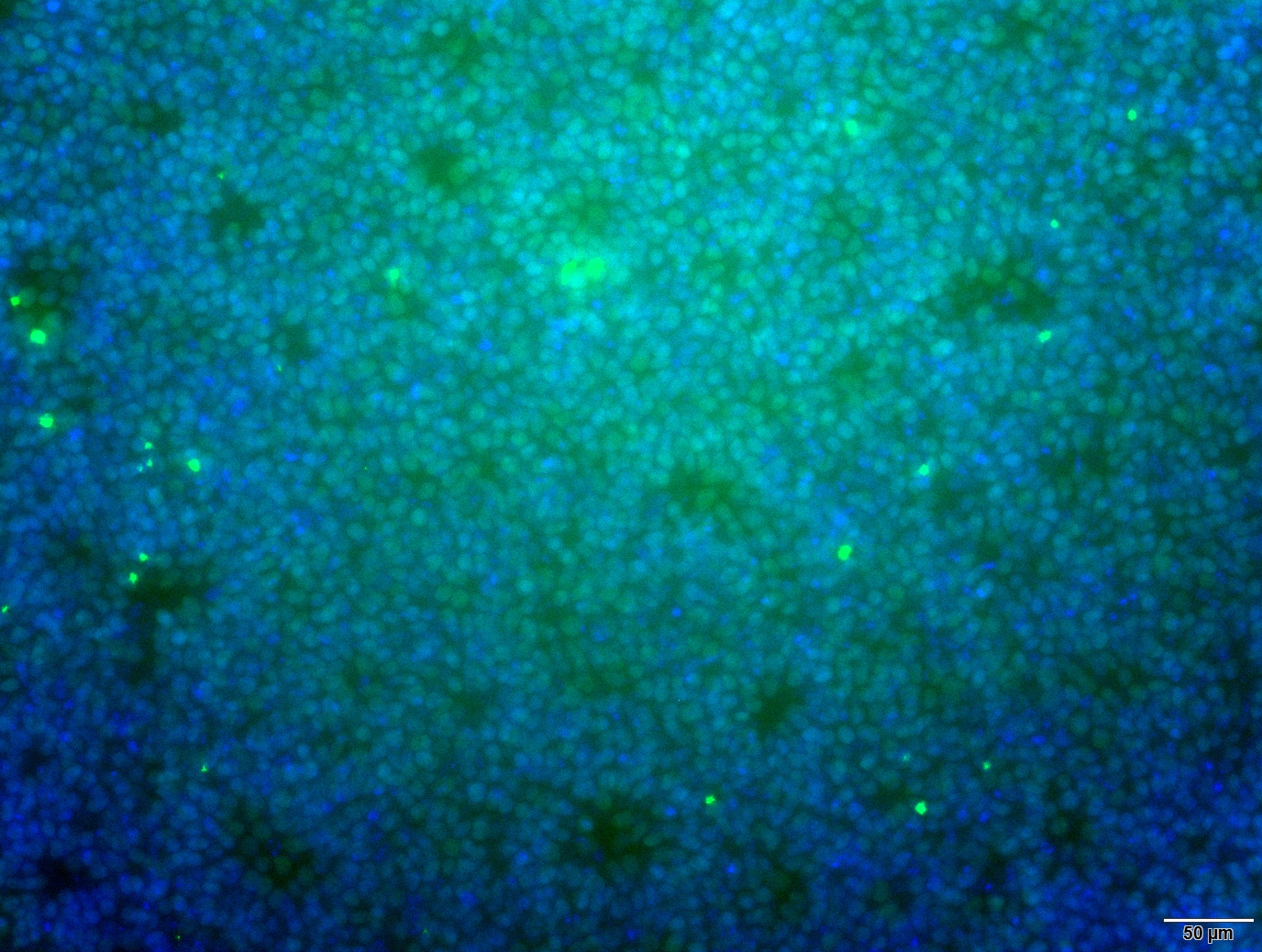
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| NANOG |
Yes |
|||||
| POU5F1 (OCT-4) |
Yes |
|||||
| SOX2 |
Yes |
Differentiation Potency
In vitro directed differentiation
Protocol or reference
Microbiology / Virus Screening |
|
| Mycoplasma | Negative |
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|
Genetic Modification
| Disease/phenotype related modifications |
|


Login to share your feedback, experiences or results with the research community.