iPSC-WFS1-#1
IMBPASi001-A
General
Cell Line |
|
| hPSCreg name | IMBPASi001-A |
| Cite as: | IMBPASi001-A (RRID:CVCL_YT29) |
| Alternative name(s) |
iPSC-WFS1-#1
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines | No similar lines found. |
| Last update | 14th April 2022 |
| Notes | Wolfram Syndrome manifests in insulin-independent diabetes, diabetes insipidus, optic nerve atrophy, neurodegeneration, and endocrine disorders. It results in death in middle adulthood. The iPSCs line can be applied as a tool for disease modeling, and identification of therapeutic drugs for rescuing a normal phenotype. |
| User feedback | |
Provider |
|
| Generator | Institute of Medical Biology of Polish Academy of Sciences (IMBPAS) |
| Owner | Institute of Medical Biology of Polish Academy of Sciences (IMBPAS) |
| Distributors | |
| Derivation country | Poland |
External Databases |
|
| BioSamples | SAMEA6877873 |
| Cellosaurus | CVCL_YT29 |
| Wikidata | Q94316000 |
General Information |
|
| Publications |
|
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: not allowed
Commercial use: not allowed
Additional restrictions:
Distribution of the cell line according to the Coriell Institute for Medical Research policy. |
Donor Information
General Donor Information |
|
| Sex | female |
| Age of donor (at collection) | 10-14 |
| Ethnicity | Caucasian |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
| Disease associated phenotypes |
|
Karyotyping (Donor) |
|
| Has the donor karyotype been analysed? |
No
|
External Databases (Donor) |
|
| BioSamples | SAMEA6877872 |
Ethics
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Has the donor been informed that participation will not directly influence their personal treatment? | Yes |
| Can you provide us with a copy of the Donor Information Sheet provided to the donor? | No |
| Do you (Depositor/Provider) hold the original Donor Consent Form? | No |
| If you do not hold the Donor Consent Form, do you know who does? | No |
| Alternatives to consent are available? | No |
| Is there other documentation provided to the donor for consenting purposes? | No |
| Has the donor agreed to be re-contacted? | Unknown |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | pseudonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| * Does consent pertain to a specific research project? | No |
| Does consent permit unforeseen future research, without further consent? | Yes |
| Does consent expressly prevent development of commercial products? | Yes |
| Does consent expressly permit storage of donated embryo/tissue for an unlimited time? | No |
| Does consent expressly permit storage of cells derived from the donated embryo/tissue for an unlimited time? | No |
| Does consent prevent the DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
| Does consent prevent CELLS DERIVED FROM THE DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
Does consent permit research by | |
| an academic institution? | Yes |
| a public organisation? | Yes |
| a non-profit company? | Yes |
| a for-profit corporation? | No |
| How may genetic information associated with the cell line be accessed? | Controlled Access |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Does the consent anticipate that the donor will be notified of results or outcomes of any research involving the donated samples or derived cells? | No |
| Does the consent permit the donor, upon withdrawal of consent, to stop the use of the derived cell line(s) that have already been created from donated samples? | Yes |
| Does the consent permit the donor, upon withdrawal of consent, to stop delivery or use of information and data about the donor? | Yes |
| Does consent permit access to medical records of the donor? | No |
| Does consent permit access to any other source of information about the clinical treatment or health of the donor? | No |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | US National Institutes of Health |
| Approval number | CC-GM-15-004 |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the PROPOSED PROJECT, involving use of donated embryo/tissue or derived cells? | No |
| Are you aware of any further constraints on the use of the donated embryo/tissue or derived cells? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | Addgene |
hIPSC Derivation
General |
|
| Source cell line name | GM01610 |
| Source cell type |
Any skin fibroblast that is part of some dermis.
|
| Age of donor (at collection) | 10-14 |
| Source cell line vendor | Coriell Institute for Medical Research |
| Passage number reprogrammed | 5 |
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Episomal |
| Genes | |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
| Notes on reprogramming vector detection | PCR reaction was used to detect reprogramming vectors in the isolated genomic DNA, using episomal plasmid-specific primers. After passage 20, the episomal vectors were found absent, indicating that these iPS cells are essentially integration-free. |
| Files and images showing reprogramming vector expressed or silenced | |
Vector free reprogramming |
|
Other |
|
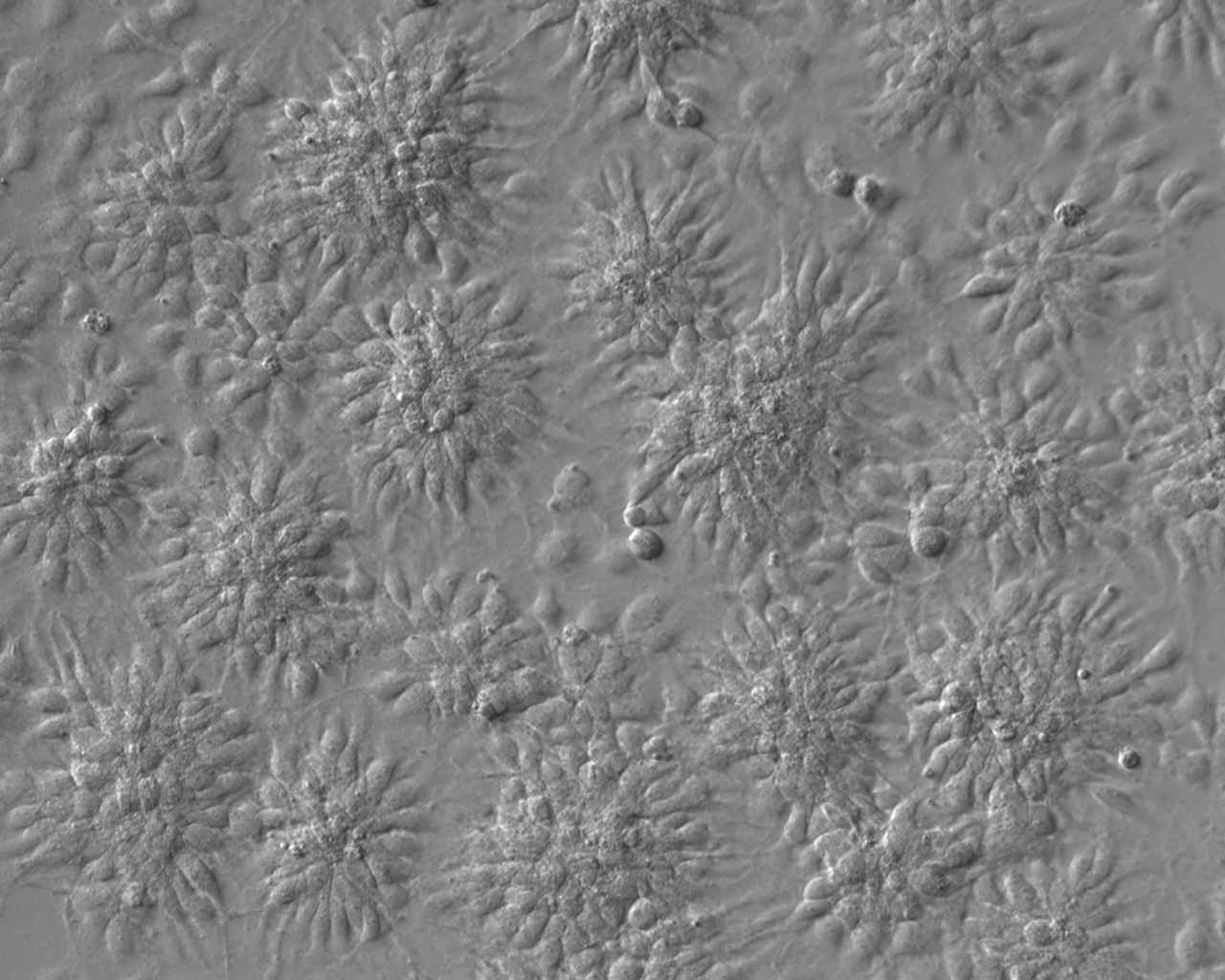
| Selection criteria for clones | iPSCs from a patient affected by Wolfram Syndrome were generated from fibroblast cells of 13-years old female (GM01610, deposited at Coriell Institute repository). These cells were reprogrammed with the use of EBV-derived oriP/EBNA-1 episomal system carrying Oct3/4, Sox2, Klf4, Lin28 transcription factors, and shRNA against p53 (pCXLE-hOCT3/4-shp53-F, pCXLE-hSK pCXLE-hUL and pCXWB-EBNA1). One of the clones of reprogrammed cells, showing ESC-like morphology was further expanded and characterized. |
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
EDTA
|
| O2 Concentration | 21 % |
| CO2 Concentration | 5 % |
| Medium |
Essential 8™
|
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | Yes |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | Yes |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | Yes |
Characterisation
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| SOX2 |
Yes |
|
||||
| NANOG |
Yes |
|||||
| TRA 1-60 |
Yes |
|||||
| TRA 1-81 |
Yes |
|||||
| POU5F1 (OCT-4) |
Yes |
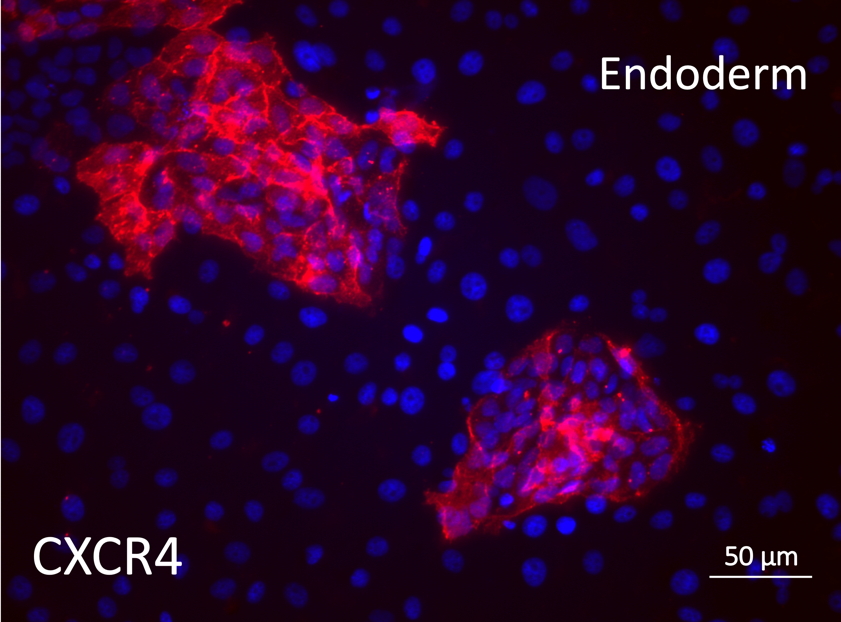
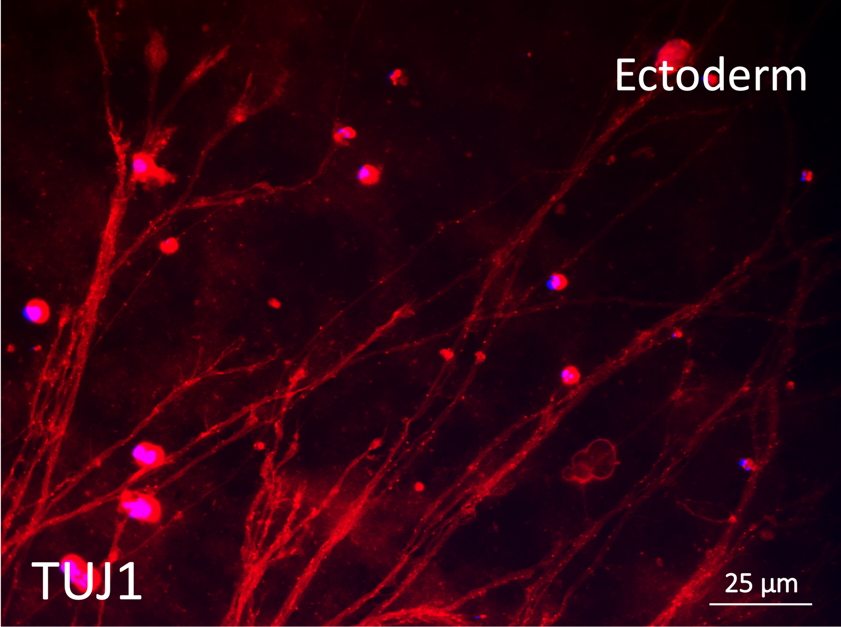
Differentiation Potency
Microbiology / Virus Screening |
|
| Mycoplasma | Negative |
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|






Login to share your feedback, experiences or results with the research community.