Phx_SRCAP_g3_1200_7
MHHi001-A-13
General
Donor Information
General Donor Information |
|
| Sex | female |
| Ethnicity | Caucasian |
Phenotype and Disease related information (Donor) |
|
| Diseases | No disease was diagnosed.
|
| Family history | N/A |
| Is the medical history available upon request? | no |
| Is clinical information available? | no |
Karyotyping (Donor) |
|
| Has the donor karyotype been analysed? |
Unknown
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
No
|
External Databases (Donor) |
|
| BioSamples | SAMEA4564585 |
Ethics
Also have a look at the ethics information for the parental line
MHHi001-A
.
| Is there an MTA available for the cell line? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | |
| Are you aware of any constraints on the use or distribution of the cell line from the owner or any parties identified in the query above? | No |
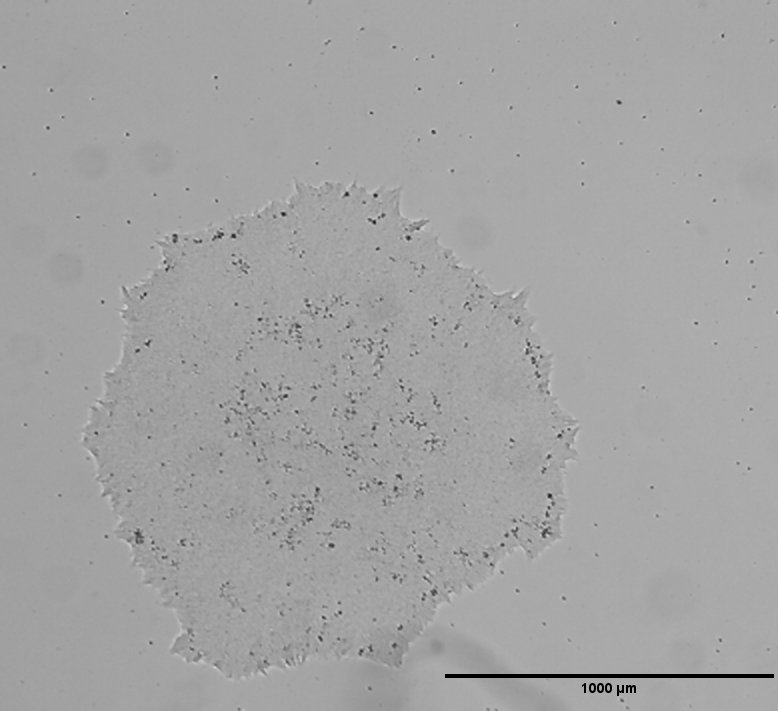
hIPSC Derivation
General |
|
|
The source cell information can be found in the parental cell line
MHHi001-A.
|
|
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Sendai virus |
| Is reprogramming vector detectable? |
No |
| Methods used |
RT-PCR
|
| Notes on reprogramming vector detection | rtPCR with Primer specific for genes on SeV vector and vector backbone, GAPDH as positive control |
| Files and images showing reprogramming vector expressed or silenced | |
Vector free reprogramming |
|
Other |
|
| Derived under xeno-free conditions |
Unknown |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Matrigel/Geltrex | ||||||
| Feeder cells |
No |
||||||
| Passage method | Mechanically | ||||||
| CO2 Concentration | 5 % | ||||||
| Medium |
mTeSR™ 1
Supplements
|
||||||
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | No |
||||||
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
||||||
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | Unknown |
Characterisation
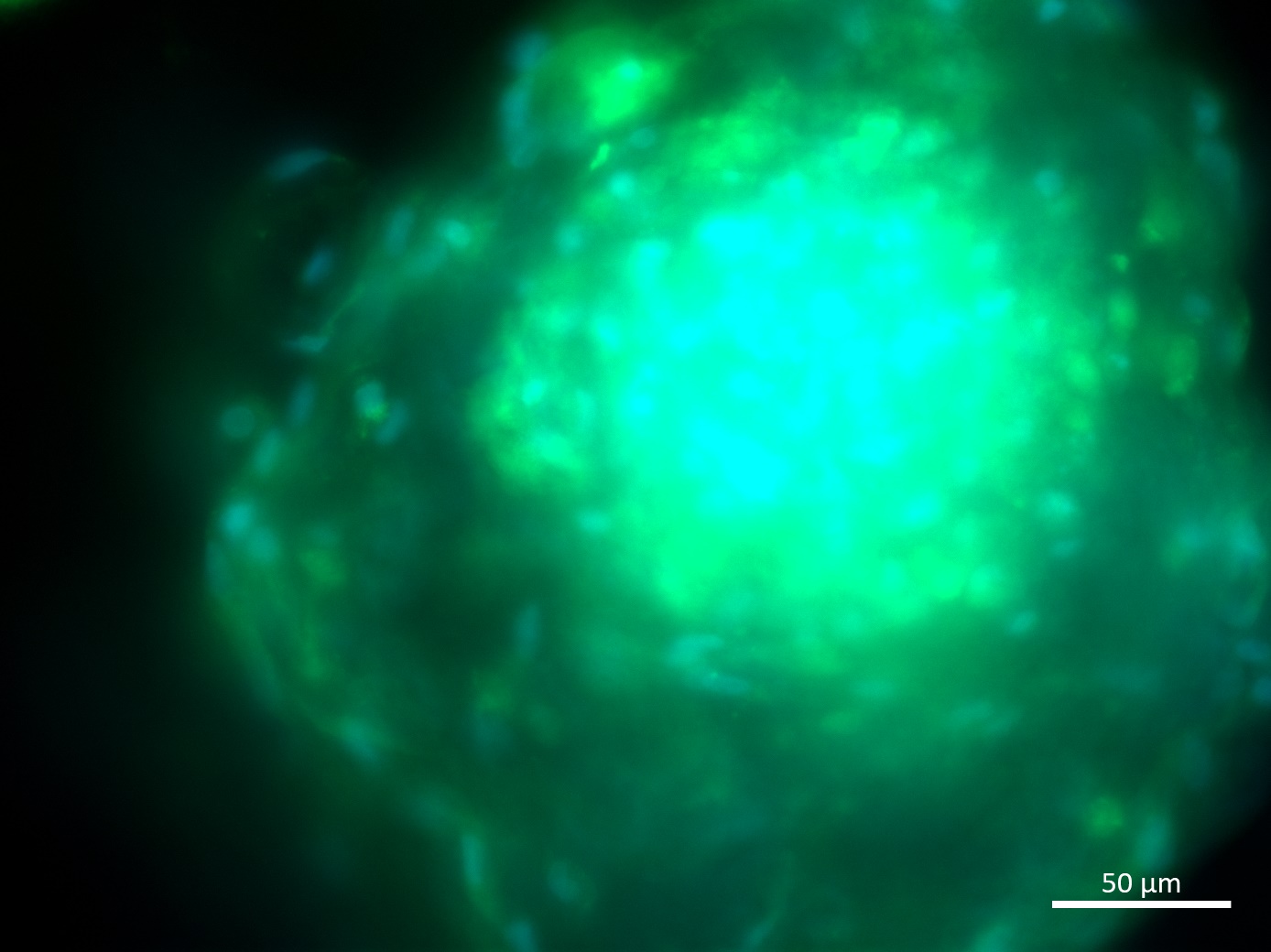
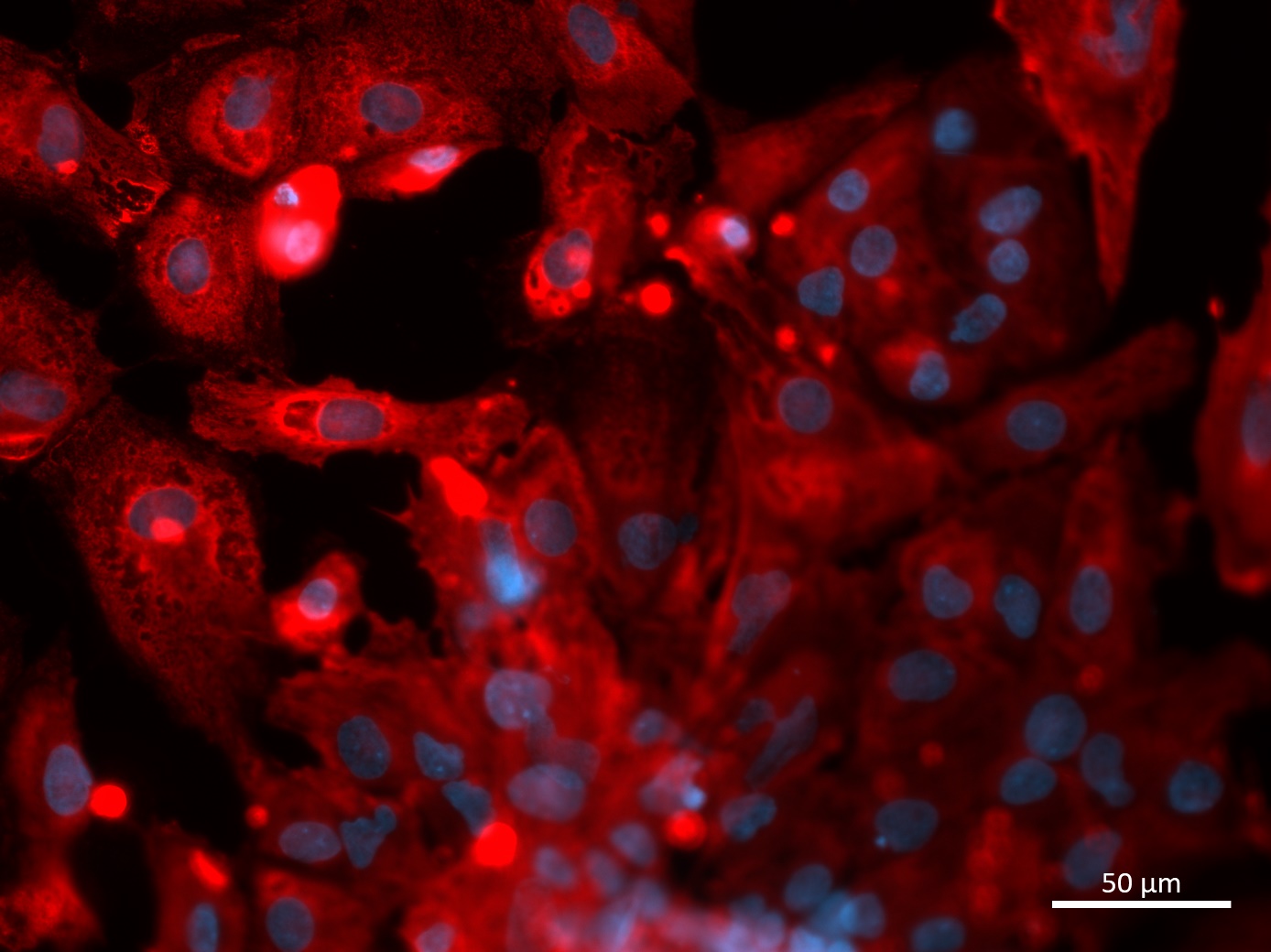
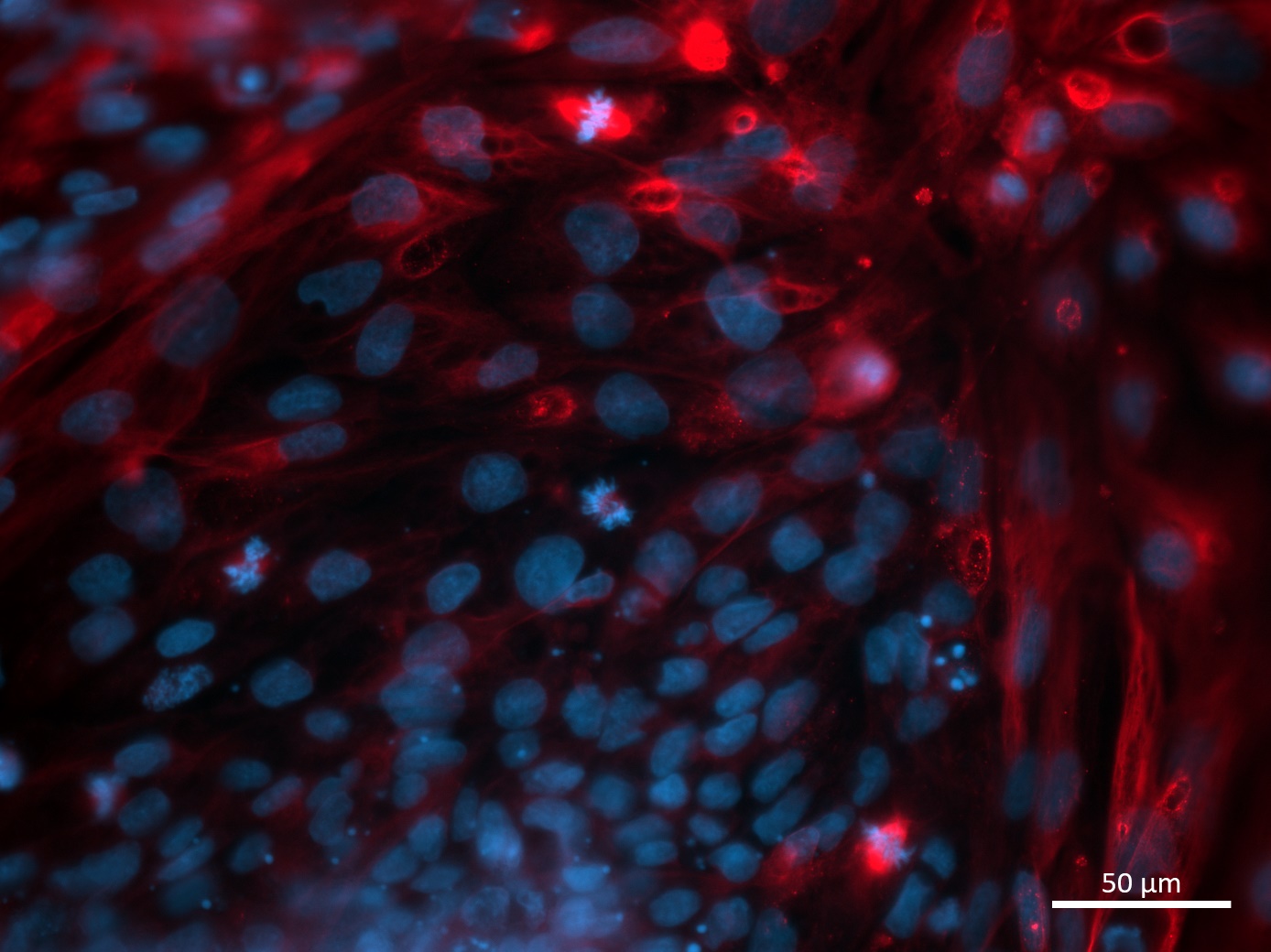
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| POU5F1 (OCT-4) |
Yes |
|||||
| SOX2 |
Yes |
|||||
| TRA 1-60 |
Yes |
|||||
| NANOG |
Yes |
Differentiation Potency
Microbiology / Virus Screening |
|
| Mycoplasma | Negative |
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|
Genetic Modification
| Disease/phenotype related modifications |
|


Login to share your feedback, experiences or results with the research community.