069
VCCRIi002-A
General
Cell Line |
|
| hPSCreg name | VCCRIi002-A |
| Cite as: | VCCRIi002-A (RRID:CVCL_D0PE) |
| Alternative name(s) |
069
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines |
|
| Last update | 12th December 2023 |
| User feedback | |
Provider |
|
| Generator | Victor Chang Cardiac Research Institute (VCCRI) |
| Owner | Victor Chang Cardiac Research Institute (VCCRI) |
| Distributors | |
| Derivation country | Australia |
External Databases |
|
| BioSamples | SAMEA114465051 |
| Cellosaurus | CVCL_D0PE |
| Wikidata | Q123033768 |
General Information |
|
| Publications | |
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: not allowed
Commercial use: not allowed
Additional restrictions:
Restrictions subject to Ethics |
Donor Information
General Donor Information |
|
| Sex | female |
| Ethnicity | Caucasian |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
| Family history | Sibling with SCAD |
| Is the medical history available upon request? | Available in accordance with ethics |
| Is clinical information available? | Available in accordance with ethics |
Karyotyping (Donor) |
|
| Has the donor karyotype been analysed? |
No
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
|
Donor Relations |
|
| All cell lines of this donor's relatives |
Has sister:
|
External Databases (Donor) |
|
| BioSamples | SAMEA114465044 |
Ethics
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Has the donor been informed that participation will not directly influence their personal treatment? | Yes |
| Can you provide us with a copy of the Donor Information Sheet provided to the donor? | Yes |
| Do you (Depositor/Provider) hold the original Donor Consent Form? | Yes |
| Alternatives to consent are available? | No |
| Is there other documentation provided to the donor for consenting purposes? | No |
| Confirm that consent was obtained by a qualified professional | Yes |
| Has the donor agreed to be re-contacted? | Yes |
| Has the donor been informed about how her/his data will be protected? | Yes |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | pseudonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| * Does consent expressly prevent the derivation of pluripotent stem cells? | No |
| Does consent expressly prevent development of commercial products? | No |
| Does consent expressly prevent financial gain from any use of the donated embryo/tissue, including any product made from it? | No |
| Does consent expressly permit storage of donated embryo/tissue for an unlimited time? | No |
| Does consent prevent CELLS DERIVED FROM THE DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
Does consent permit research by | |
| an academic institution? | Yes |
| a public organisation? | No |
| a non-profit company? | No |
| a for-profit corporation? | No |
| How may genetic information associated with the cell line be accessed? | Controlled Access |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | St Vincent's Hospital Human Research Ethics Committee |
| Approval number | HREC/16/SVH/338 |
| Do you have obligations to third parties in regard to the use of the cell line? | No |
| Are you aware of any further constraints on the use of the donated embryo/tissue or derived cells? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | |
hIPSC Derivation
General |
|
| Source cell type |
A peripheral blood cell with a single nucleus. This category includes lymphocytes and monocytes.
Synonyms
|
| Source cell origin |
A liquid tissue; its major function is to transport oxygen throughout the body. It also supplies the tissues with nutrients, removes waste products, and contains various components of the immune system defending the body against infection. Several hormones also travel in the blood.
Synonyms
|
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Sendai virus |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
Vector free reprogramming |
|
Other |
|
| Derived under xeno-free conditions |
Yes |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
EDTA
|
| O2 Concentration | 18 % |
| CO2 Concentration | 5 % |
| Medium |
mTeSR™ Plus
|
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | Yes |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | Yes |
Characterisation
Analysis of Undifferentiated Cells
Self-renewal
Positive
Endoderm
Negative
Mesoderm
Negative
Ectoderm score
Negative
Scorecard
069_Scorecard_2_2020-10-7_93630_2020-10-06_094722.pdf
Scorecard for Line 069
Differentiation Potency
In vitro directed differentiation
Scorecard
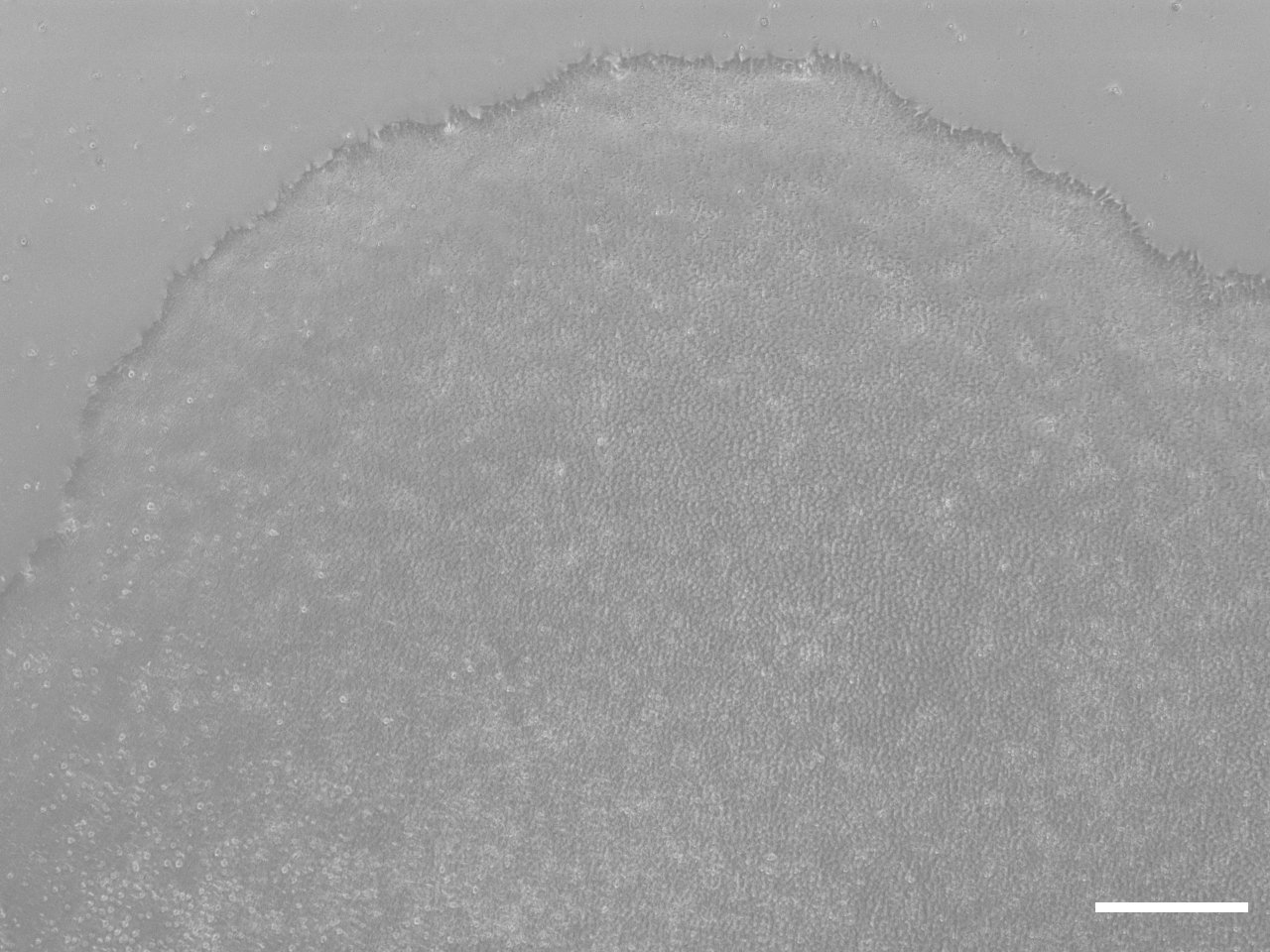
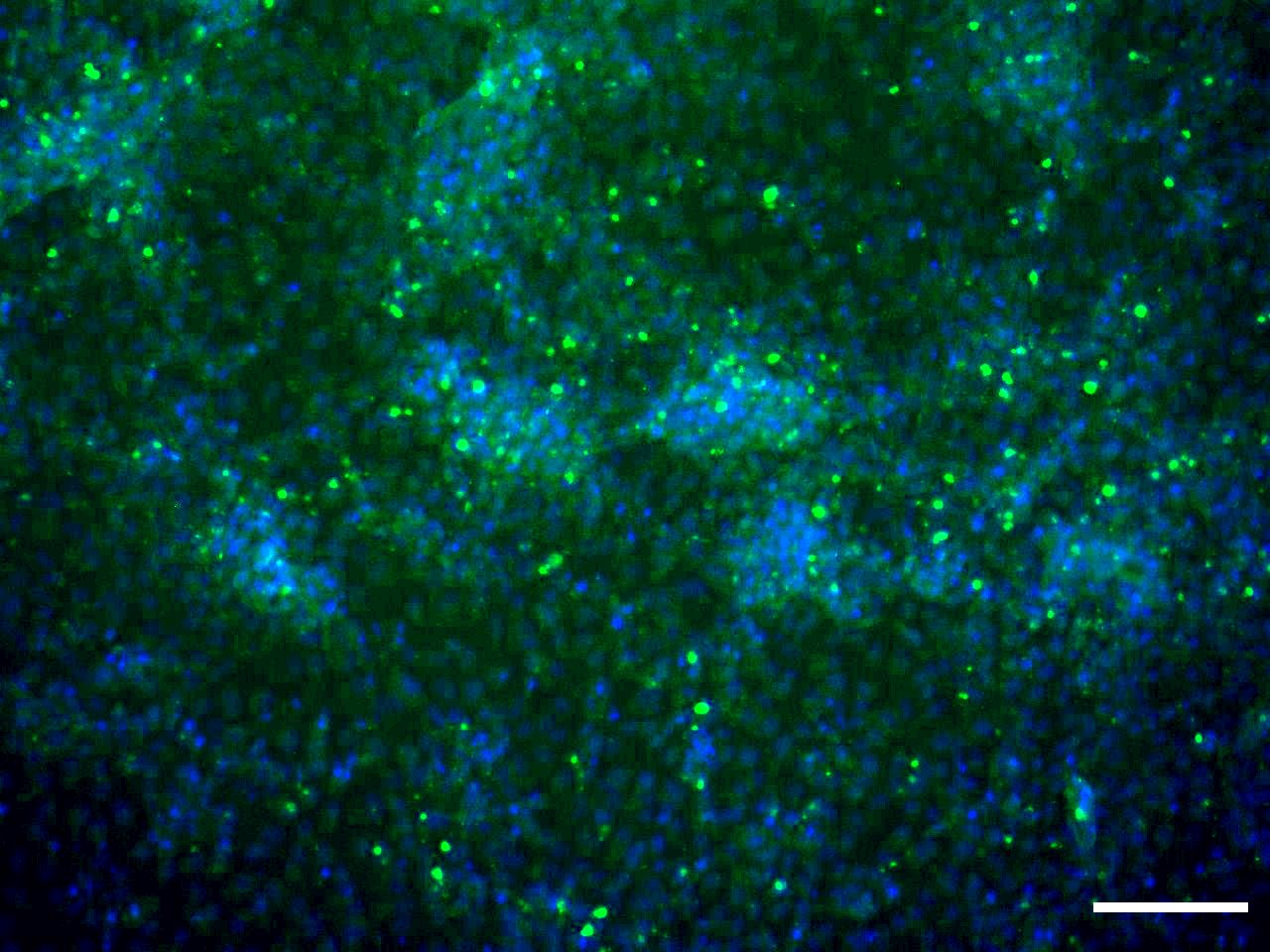
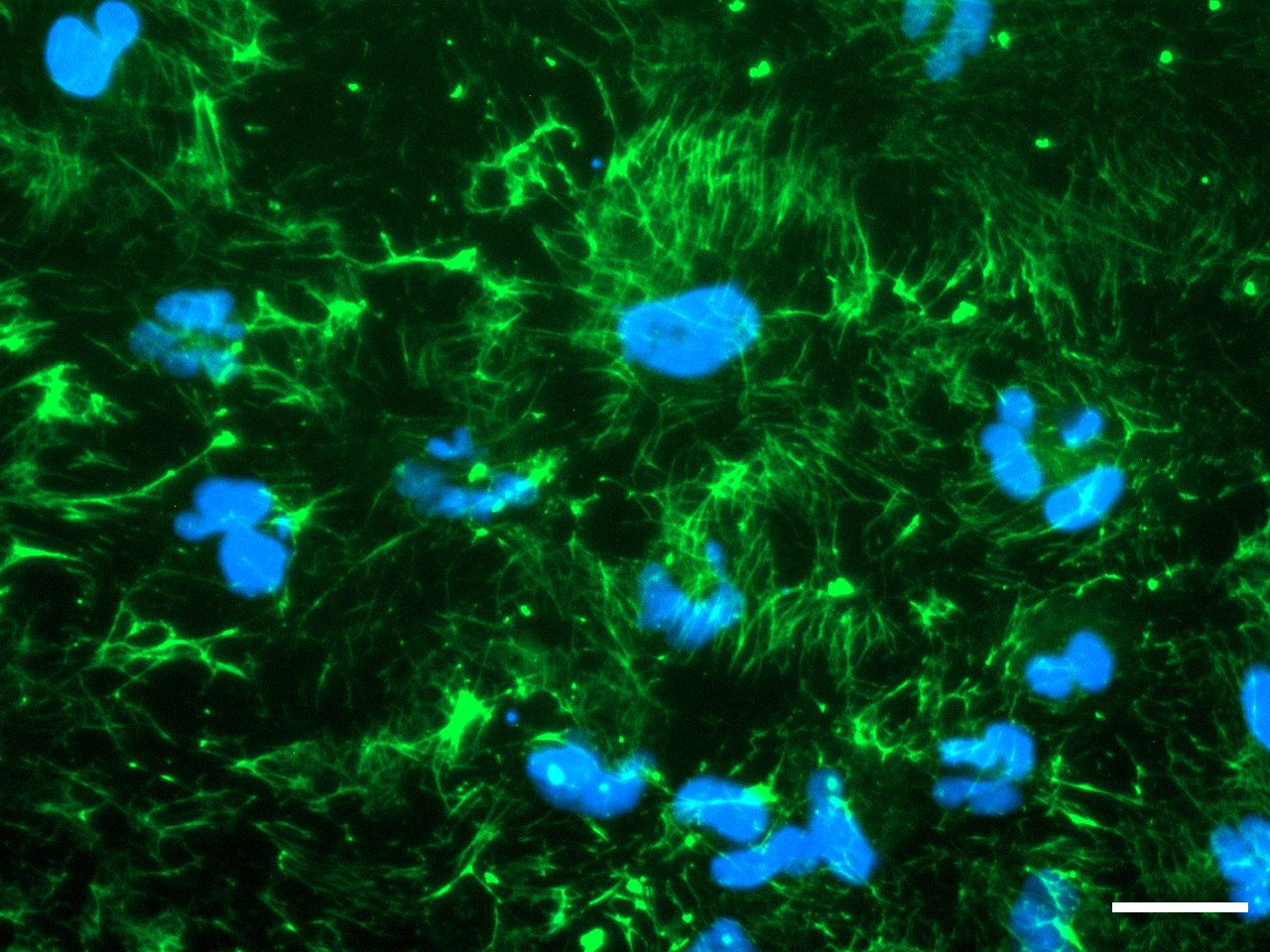
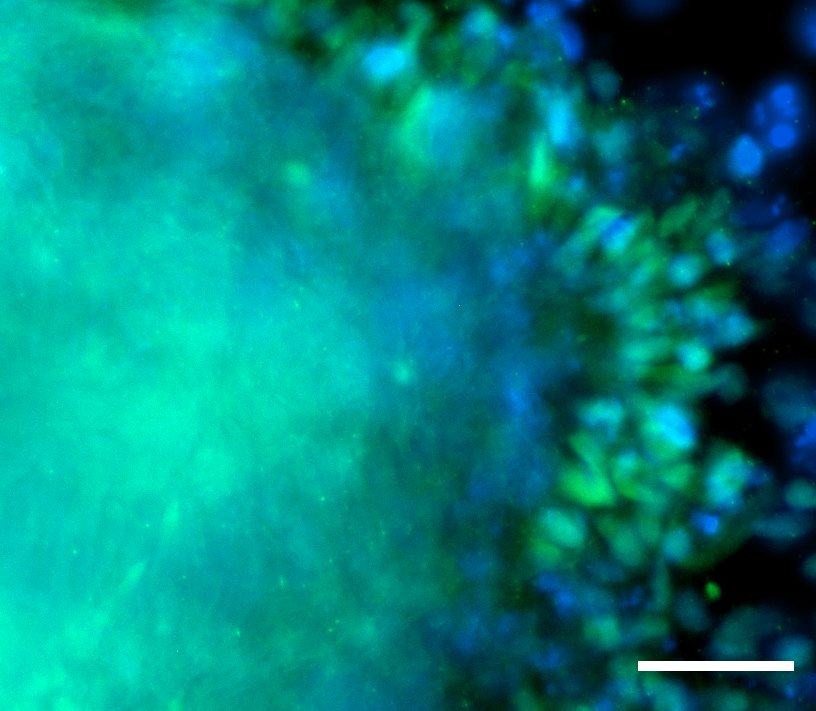
Morphology
Supplementary Figure 3 Scorecard.pdf
Scorecard Line 69 Trilineage
Protocol or reference
Methods for hPSCreg.JPG
Methods from hPSCreg
In vitro directed differentiation
Scorecard
| Marker | Expressed |
| Brachyury |
Yes |
Morphology
Supplementary Figure 3 Scorecard.pdf
Scorecard
Protocol or reference
Methods for hPSCreg.JPG
Methods from hPSCreg
In vitro directed differentiation
Scorecard
| Marker | Expressed |
| PAX6 |
Yes |
Morphology
Supplementary Figure 3 Scorecard.pdf
Scorecard
Protocol or reference
Methods for hPSCreg.JPG
Methods from hPSCreg
Microbiology / Virus Screening |
|
| HIV 1 | Negative |
| HIV 2 | Negative |
| Hepatitis B | Negative |
| Hepatitis C | Negative |
| Mycoplasma | Negative |
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
|


Login to share your feedback, experiences or results with the research community.