HS293
KIe005-A
General
Donor Information
General Donor Information |
|
Phenotype and Disease related information (Donor) |
|
| Diseases | |
Ethics
| Alternatives to consent are available? | Yes |
| Alternatives to consent | |
| Alternative consent approval number | |
| How may genetic information associated with the cell line be accessed? | |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | Regional ethical committee |
| Approval number |
hESC Derivation
| Date of derivation | 2003-02-01 |
| Embryo stage | Blastula with ICM and Trophoblast |
| PGD Embryo? |
No |
| Expansion status |
3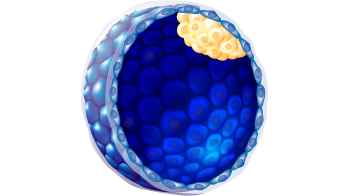
|
| ICM morphology |
B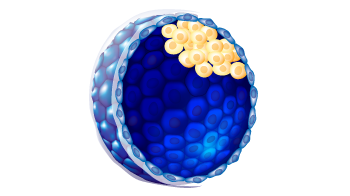
|
| Trophectoderm morphology |
b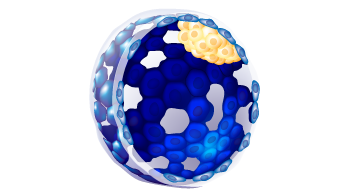
|
| ZP removal technique | Enzymatic |
| Cell isolation | Immunosurgery |
| Cell seeding | Isolated ICM |
Culture Conditions
| Surface coating | hFF | ||||||||||||
| O2 Concentration | 20 % | ||||||||||||
| CO2 Concentration | 5 % | ||||||||||||
| Medium |
Other medium:
Base medium: Knockout-DMEM
Main protein source: Knock-out serum replacement Serum concentration: 20 % Supplements
|
Characterisation
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| GCTM-2 |
Yes |
|
||||
| NANOG |
Yes |
|
|
|||
| POU5F1 (OCT-4) |
Yes |
|
|
|||
| SSEA-1 |
No |
|
|
|
||
| SSEA-4 |
Yes |
|
|
|
||
| TRA 1-60 |
Yes |
|
||||
| TRA 1-81 |
Yes |
|
Differentiation Potency
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
46XY
Passage number: 49
|
Other Genotyping (Cell Line) |
|


Login to share your feedback, experiences or results with the research community.