JTUi002-A
General
Donor Information
General Donor Information |
|
| Sex | male |
| Age of donor (at collection) | 5-9 |
| Ethnicity | Han nationality |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
| Family history | His both pareants are hearing impaired. |
| Is the medical history available upon request? | Yes. |
| Is clinical information available? | Yes. |
Karyotyping (Donor) |
|
| Has the donor karyotype been analysed? |
Yes
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
Exome sequencing
|
External Databases (Donor) |
|
| BioSamples | SAMEA6613350 |
Ethics
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Has the donor been informed that participation will not directly influence their personal treatment? | Yes |
| Can you provide us with a copy of the Donor Information Sheet provided to the donor? | No |
| Do you (Depositor/Provider) hold the original Donor Consent Form? | Yes |
| Alternatives to consent are available? | No |
| Is there other documentation provided to the donor for consenting purposes? | No |
| Confirm that consent was obtained by a qualified professional | Yes |
| Has the donor agreed to be re-contacted? | Unknown |
| Has the donor been informed about how her/his data will be protected? | Yes |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | anonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| * Does consent pertain to a specific research project? | No |
| Does consent permit unforeseen future research, without further consent? | No |
| Does the consent permit uses of donated embryo/tissue or derived cell line intended for clinical treatment or human applications? | No |
| Does consent expressly prevent development of commercial products? | Yes |
| Does consent expressly prevent financial gain from any use of the donated embryo/tissue, including any product made from it? | Yes |
| Does consent expressly permit storage of donated embryo/tissue for an unlimited time? | No |
| Does consent expressly permit storage of cells derived from the donated embryo/tissue for an unlimited time? | No |
| Does consent prevent the DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
| Does consent prevent CELLS DERIVED FROM THE DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
Does consent permit research by | |
| an academic institution? | Yes |
| a public organisation? | Yes |
| a non-profit company? | No |
| a for-profit corporation? | No |
| Does consent expressly permit collection of genetic information? | Yes |
| Does consent expressly permit storage of genetic information? | Yes |
| Does consent prevent dissemination of genetic information? | Yes |
| Has the donor been informed that their donated biosample or derived cells may be tested for the presence of microbiological agents / pathogens? | Yes |
| Has the donor consented to receive information discovered during use of donated embryo/tissue that has significant health implications for the donor? | Yes |
| How may genetic information associated with the cell line be accessed? | Controlled Access |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | Ethics Committee of Shanghai Sixth People's Hospital |
| Approval number | 2017-136 |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the PROPOSED PROJECT, involving use of donated embryo/tissue or derived cells? | Yes |
| Name of accrediting authority involved? | Ethics Committee of Shanghai Sixth People's Hospital |
| Approval number | 2017-136 |
| Is there an MTA available for the cell line? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | |
hIPSC Derivation
General |
|
| Source cell type |
Synonyms
|
| Age of donor (at collection) | 5-9 |
| Passage number reprogrammed | 7 |
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Episomal |
| Genes | |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
| Vector map | |
Vector free reprogramming |
|
Other |
|
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Matrigel/Geltrex |
| Feeder cells |
No |
| Passage method |
Enzyme-free cell dissociation
EDTA
|
| CO2 Concentration | 5 % |
| Medium |
mTeSR™ 1
|
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | Yes |
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | Yes |
Characterisation
Analysis of Undifferentiated Cells
Score:
| Marker | Present | Absent |
| mCpG | ||
| OCT4 |
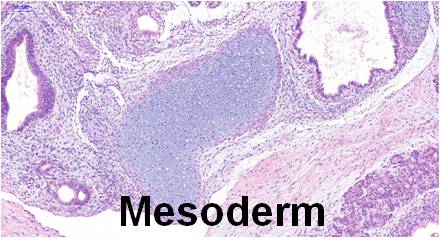
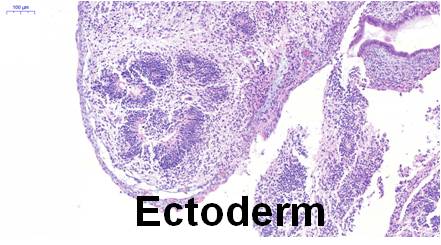
Differentiation Potency
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
|



Login to share your feedback, experiences or results with the research community.