PD58-7
ICGi053-B
General
Cell Line |
|
| hPSCreg name | ICGi053-B |
| Cite as: | ICGi053-B |
| Alternative name(s) |
PD58-7
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines |
EDi001-A-2 (AST23-1KO-3, AST22-1KO-3, AST-23_SCAKO Clone 3, AST-22_SNCAKO Clone 3) Donor's gene variants: SNCA, SNCA, SNCA, SNCA Donor diseases: Parkinson disease EDi001-A-3 (AST23_SNCAKO Clone 1, AST22-1KO-1, AST23-1KO-1, AST22_SNCAKO Clone 1) Donor's gene variants: SNCA, SNCA, SNCA, SNCA Donor diseases: Parkinson disease EDi001-A-4 (AST22-2KO-6, AST23_SNCAKO Clone 6, AST22_SNCAKO Clone 6, AST23-2KO-6) Donor's gene variants: SNCA, SNCA, SNCA, SNCA Donor diseases: Parkinson disease CBIGi007-A-1 (IPSC0009, TMEM175 Q65Q homozygous correction) Donor's gene variants: TMEM175, TMEM175 Donor diseases: Parkinson Disease CBIGi007-A-2 (IPSC0010, TMEM175 P65P homozygous disease) Donor's gene variants: TMEM175, TMEM175 Donor diseases: Parkinson Disease CBIGi009-A-1 (IPSC0013, TMEM175 M393T homozygous correction) Donor's gene variants: TMEM175, TMEM175 Donor diseases: Parkinson Disease CBIGi013-A-1 (LRRK2 M1646T correction, IPSC0018) Donor's gene variants: LRRK2, LRRK2 Donor diseases: Parkinson Disease CBIGi027-A-1 (IPSC0039, LRRK2 G2385R (CBIGi027) correction) Donor's gene variants: LRRK2, LRRK2 Donor diseases: Parkinson Disease STBCi004-B-1 (SFC832-03-06 LRRK2WT/WT C47) Donor's gene variants: LRRK2 Donor diseases: Parkinson disease CBIGi005-A (IPSC0003, LRRK2 G2019S) Donor's gene variants: LRRK2, LRRK2 Donor diseases: Parkinson Disease CBIGi007-A (IPSC0008, TMEM175 Q65P heterozygous) Donor's gene variants: TMEM175, TMEM175 Donor diseases: Parkinson Disease CBIGi009-A (IPSC0012, TMEM175 M393T homozygous) Donor's gene variants: TMEM175, TMEM175 Donor diseases: Parkinson Disease CBIGi013-A (IPSC0017, LRRK2 M1646T (heterozygous)) Donor's gene variants: LRRK2, LRRK2 Donor diseases: Parkinson Disease CBIGi015-A (IPSC0020, LRRK2 N551K-R1398H-K1423K (protective haplotype)) Donor's gene variants: LRRK2, LRRK2 Donor diseases: Parkinson Disease CBIGi016-A (IPSC0021, Parkin homozygous deletion between exon 3 and 4) Donor's gene variants: PRKN, PRKN Donor diseases: Parkinson Disease CBIGi009-B (TMEM175 M393T homozygous (CBIGi009), IPSC0033) Donor's gene variants: TMEM175, TMEM175 Donor diseases: Parkinson Disease CBIGi027-A (LRRK2 G2385R (CBIGi027), IPSC0037) Donor's gene variants: LRRK2, LRRK2 Donor diseases: Parkinson Disease |
| Last update | 22nd July 2024 |
| User feedback | |
Provider |
|
| Generator | Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences (ICG) |
External Databases |
|
| BioSamples | SAMEA115839987 |
General Information |
|
| Publications | |
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: not allowed
Commercial use: not allowed
|
Donor Information
General Donor Information |
|
| Sex | female |
| Age of donor (at collection) | 35-39 |
| Ethnicity | Caucasian |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
Exome sequencing
NCBI SRR accession: SAMN42050732, BioProject PRJNA563295 Detailed analysis of the clinical exome sequencing data of the patient's PBMCs has revealed a mutation in the LGR4 gene (c.1087G>T, rs117543292). |
Donor Relations |
|
| Other cell lines of this donor | |
External Databases (Donor) |
|
| BioSamples | SAMN42050732 |
Ethics
| Has informed consent been obtained from the donor of the embryo/tissue from which the pluripotent stem cells have been derived? | Yes |
| Was the consent voluntarily given? | Yes |
| Has the donor been informed that participation will not directly influence their personal treatment? | Yes |
| Can you provide us with a copy of the Donor Information Sheet provided to the donor? | Yes |
| Do you (Depositor/Provider) hold the original Donor Consent Form? | Yes |
| Alternatives to consent are available? | No |
| Is there other documentation provided to the donor for consenting purposes? | No |
| Confirm that consent was obtained by a qualified professional | Yes |
| Has the donor agreed to be re-contacted? | Unknown |
| Has the donor been informed about how her/his data will be protected? | Yes |
| Please indicate whether the data associated with the donated material has been pseudonymised or anonymised. | pseudonymised |
| Does consent explicitly allow the derivation of pluripotent stem cells? | Yes |
| * Does consent expressly prevent the derivation of pluripotent stem cells? | No |
| * Does consent pertain to a specific research project? | No |
| Does the consent permit uses of donated embryo/tissue or derived cell line intended for clinical treatment or human applications? | No |
| Does consent expressly permit storage of donated embryo/tissue for an unlimited time? | Yes |
| Does consent expressly permit storage of cells derived from the donated embryo/tissue for an unlimited time? | Yes |
| Does consent prevent the DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
| Does consent prevent CELLS DERIVED FROM THE DONATED BIOSAMPLE from being made available to researchers anywhere in the world? | No |
Does consent permit research by | |
| an academic institution? | Yes |
| Does consent expressly permit collection of genetic information? | Yes |
| Does consent expressly permit storage of genetic information? | Yes |
| Does consent prevent dissemination of genetic information? | No |
| How may genetic information associated with the cell line be accessed? | Open Access |
| Will the donor expect to receive financial benefit, beyond reasonable expenses, in return for donating the biosample? | No |
| Does consent permit access to medical records of the donor? | No |
| Does consent permit access to any other source of information about the clinical treatment or health of the donor? | No |
| Has a favourable opinion been obtained from a research ethics committee, or other ethics review panel, in relation to the Research Protocol including the consent provisions? | Yes |
| Name of accrediting authority involved? | FSBI Federal Neurosurgical Center |
| Approval number | protocol number 1, 14/03/2017 |
| Do you have obligations to third parties in regard to the use of the cell line? | No |
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? | Addgene |
| Are you aware of any constraints on the use or distribution of the cell line from the owner or any parties identified in the query above? | No |
hIPSC Derivation
General |
|
| Source cell type |
A peripheral blood cell with a single nucleus. This category includes lymphocytes and monocytes.
Synonyms
|
| Source cell origin |
Blood drawn from a limb.
Synonyms
|
| Age of donor (at collection) | 35-39 |
| Collected in | 2022 |
| Source cell line vendor | FSBI Federal Neurosurgical Center |
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Episomal |
| Genes | |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
| Files and images showing reprogramming vector expressed or silenced | |
Vector free reprogramming |
|
| Type of used vector free reprogramming factor(s) |
None
|
Other |
|
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Gelatin | ||||||||||||||||||
| Feeder cells |
Mouse embryonic fibroblasts Cellfinder Ont Id: EFO_0004040 |
||||||||||||||||||
| Passage method |
Enzymatically
TrypLE
|
||||||||||||||||||
| O2 Concentration | 20 % | ||||||||||||||||||
| CO2 Concentration | 5 % | ||||||||||||||||||
| Medium |
Other medium:
Base medium: KnockOut DMEM
Main protein source: Knock-out serum replacement Serum concentration: 15 % Supplements
|
||||||||||||||||||
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | Yes |
||||||||||||||||||
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
||||||||||||||||||
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | Yes |
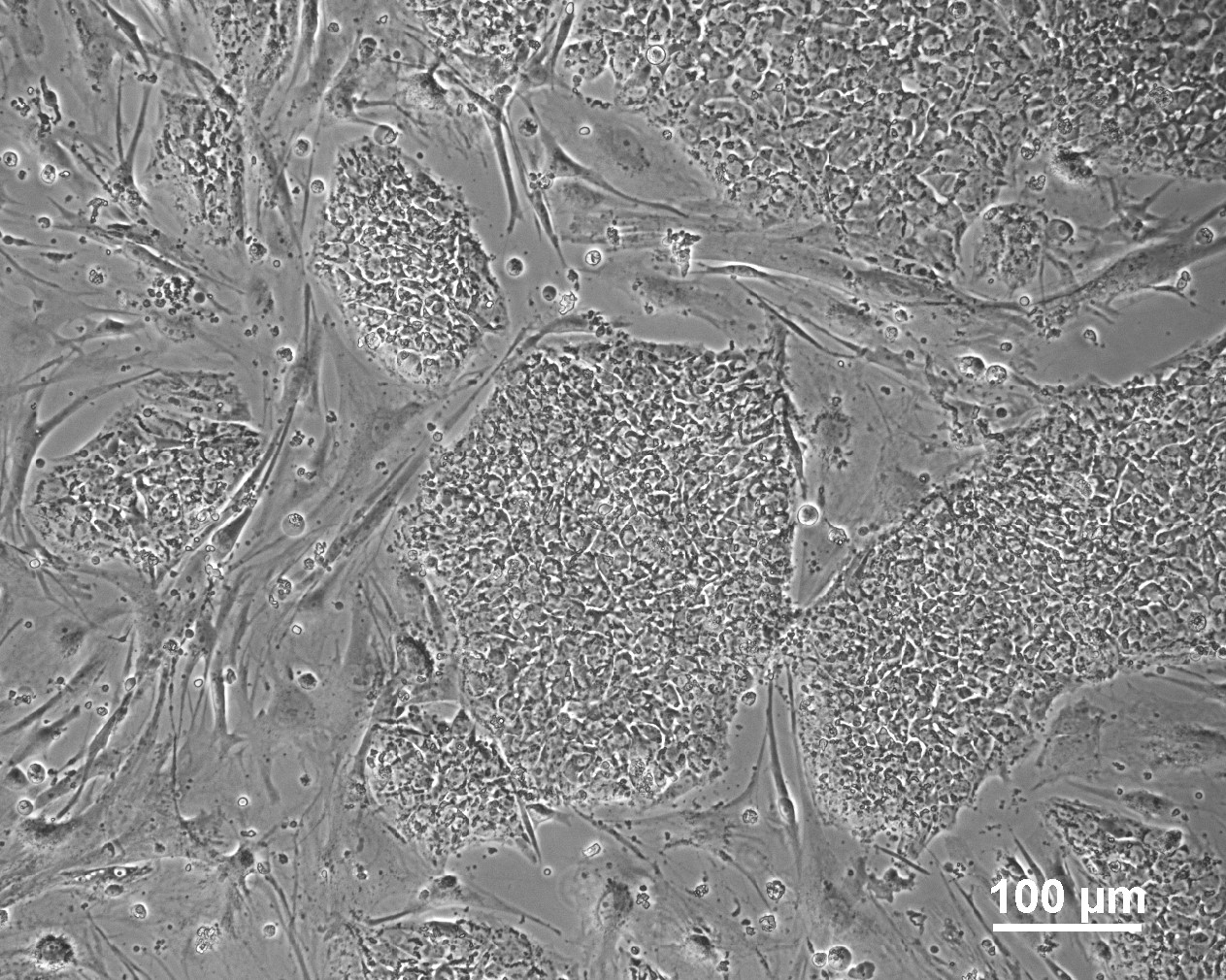
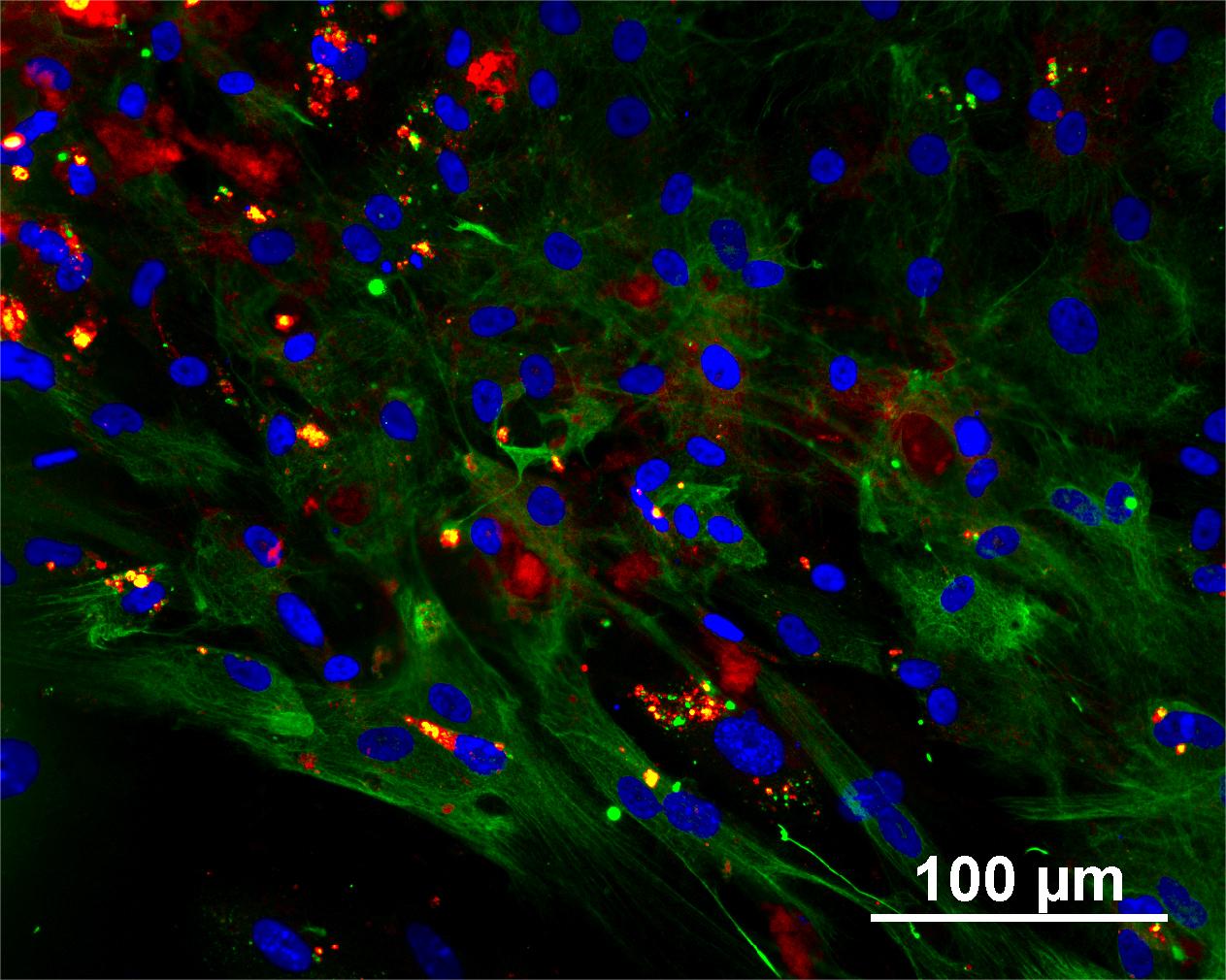
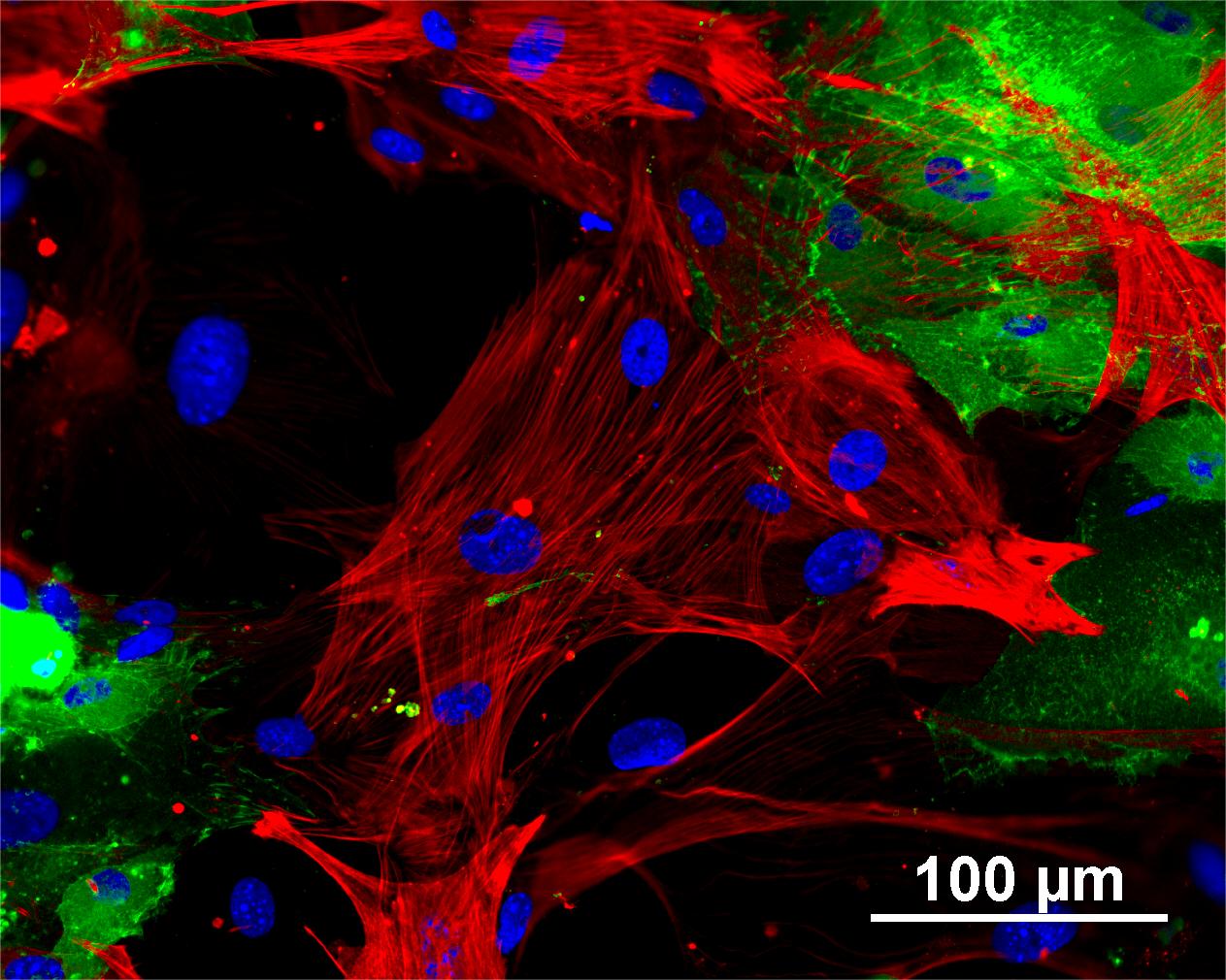
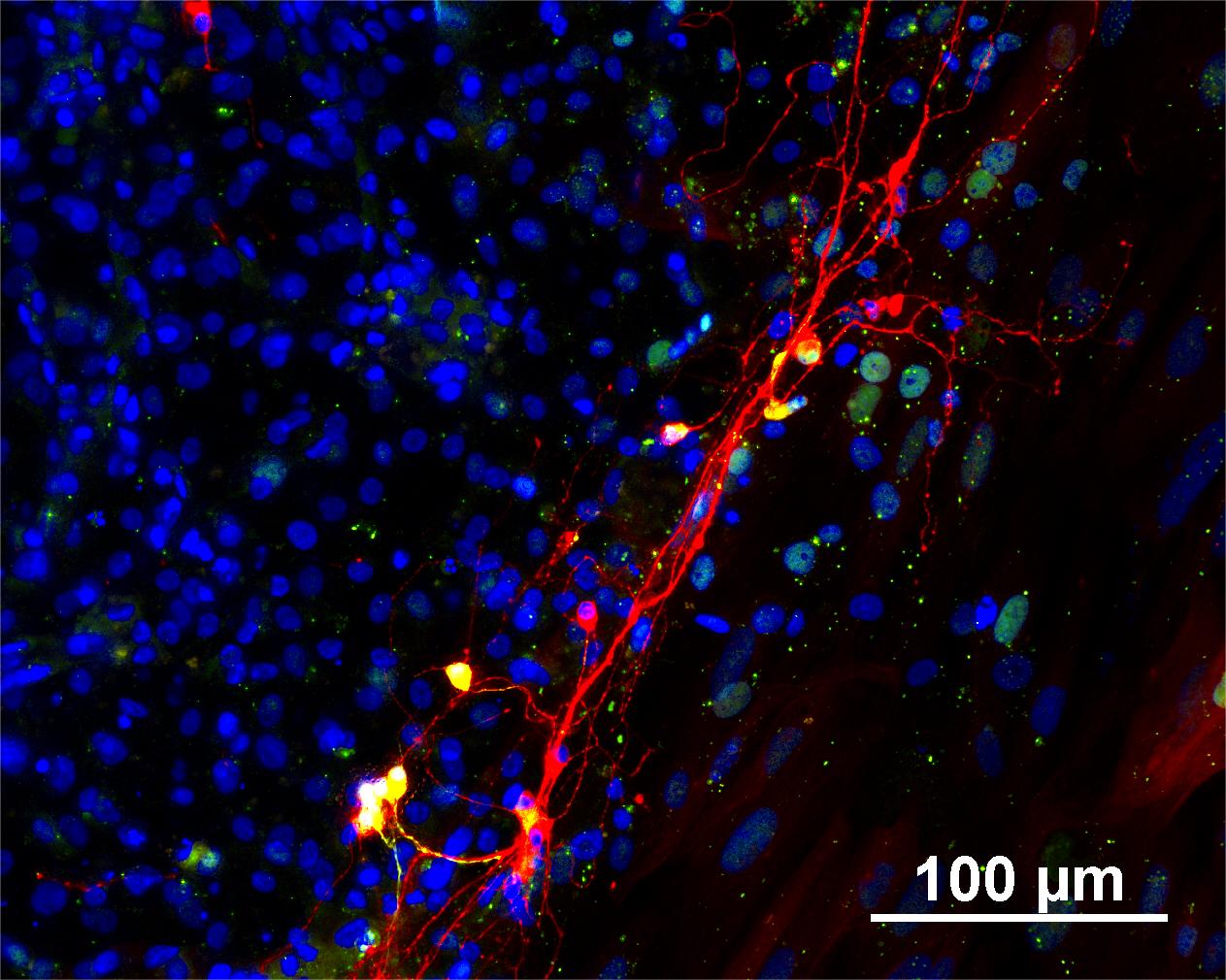
Characterisation
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| NANOG |
Yes |
|||||
| SOX2 |
Yes |
|||||
| POU5F1 (OCT-4) |
Yes |
|||||
| SSEA-4 |
Yes |
|||||
| Alkaline Phosphatase |
Yes |
Differentiation Potency
In vitro spontaneous differentiation
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
|
Other Genotyping (Cell Line) |
|


Login to share your feedback, experiences or results with the research community.