PD57-7 MT8
ICGi052-B-1
General
Cell Line |
|
| hPSCreg name | ICGi052-B-1 |
| Cite as: | ICGi052-B-1 (RRID:CVCL_E9B9) |
| Alternative name(s) |
PD57-7 MT8
|
| Cell line type | Human induced pluripotent stem cell (hiPSC) |
| Similar lines |
MRIi001-A-1 (C6-AAVS1-iCasRx) WAe007-A-5 (H7-AP1-Luciferase-GFP) MRIi003-A-7 (HK-AAVS1-iCasRx) MRIi003-A-8 (HK-AAVS1-CAG-eGFP-homo) UCSFi001-A-23 (AICS-0054-091) UCSFi001-A-1D (AICS-0120-204) UCSFi001-A-1E (AICS-0102-330) BIHi001-A-1 (iBCRT Cas9v1-3G-Kl.16) ICGi021-A-1 (K6-4fpCyto-13) ICGi021-A-2 (K6-4fpCyto-16) ICGi021-A-3 (K6-4fpCyto-19) UCSFi001-A-36 (AICS-0036-028) UCSFi001-A-82 (WTC11-SLC12A3) UCSFi001-A-12 (AICS-0036-006) CRMi003-A-3 (Proliving, GMNN-mScarletI Reporter) |
| Last update | 3rd December 2024 |
| User feedback | |
Provider |
|
| Generator | Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences (ICG) |
| Owner | Institute of Cytology and Genetics, Siberian Branch of Russian Academy of Sciences (ICG) |
| Distributors | |
| Derivation country | Russia |
External Databases |
|
| BioSamples | SAMEA117400231 |
| Cellosaurus | CVCL_E9B9 |
General Information |
|
| Publications | |
| * Is the cell line readily obtainable for third parties? |
Yes Research use: allowed
Clinical use: not allowed
Commercial use: not allowed
|
| Subclone of | |
Donor Information
General Donor Information |
|
| Sex | female |
| Ethnicity | Caucasian |
Phenotype and Disease related information (Donor) |
|
| Diseases | A disease was diagnosed.
|
| Family history | All female relatives are affected. |
Other Genotyping (Donor) |
|
| Is there genome-wide genotyping or functional data available? |
Yes
Exome sequencing
NCBI SRR accession: SAMN42050731, BioProject PRJNA563295 Detailed analysis of the clinical exome sequencing data of the patient's PBMCs has revealed a mutation in the MAPT gene (c.2013T>G, rs63750756). |
Donor Relations |
|
| Other cell lines of this donor | |
External Databases (Donor) |
|
| BioSamples | SAMN42050731 |
Ethics
Also have a look at the ethics information for the parental line
ICGi052-B
.
| For generation of the cell line, who was the supplier of any recombined DNA vectors or commercial kits used? |
hIPSC Derivation
General |
|
|
The source cell information can be found in the parental cell line
ICGi052-B.
|
|
Reprogramming method |
|
| Vector type | Non-integrating |
| Vector | Episomal |
| Is reprogramming vector detectable? |
No |
| Methods used |
PCR
|
Vector free reprogramming |
|
| Type of used vector free reprogramming factor(s) |
None
|
Other |
|
| Derived under xeno-free conditions |
No |
| Derived under GMP? |
No |
| Available as clinical grade? |
No |
Culture Conditions
| Surface coating | Gelatin | ||||||||||||||||||
| Feeder cells |
Mouse embryonic fibroblasts Cellfinder Ont Id: EFO_0004040 |
||||||||||||||||||
| Passage method |
Enzymatically
TrypLE
|
||||||||||||||||||
| O2 Concentration | 20 % | ||||||||||||||||||
| CO2 Concentration | 5 % | ||||||||||||||||||
| Medium |
Other medium:
Base medium: KnockOut DMEM
Main protein source: Knock-out serum replacement Serum concentration: 15 % Supplements
|
||||||||||||||||||
| Has Rock inhibitor (Y27632) been used at passage previously with this cell line? | Yes |
||||||||||||||||||
| Has Rock inhibitor (Y27632) been used at cryo previously with this cell line? | No |
||||||||||||||||||
| Has Rock inhibitor (Y27632) been used at thaw previously with this cell line? | Yes |
Characterisation
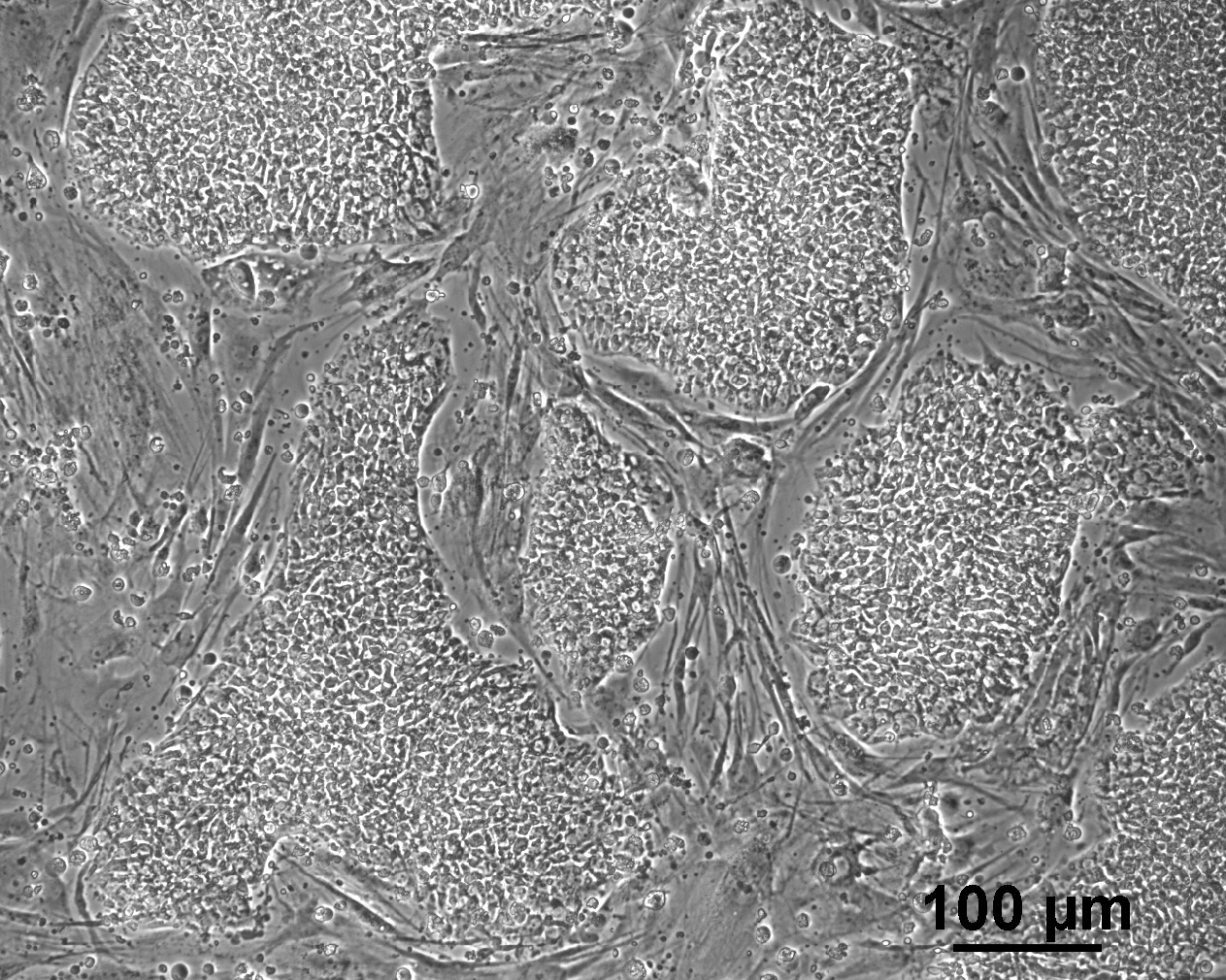
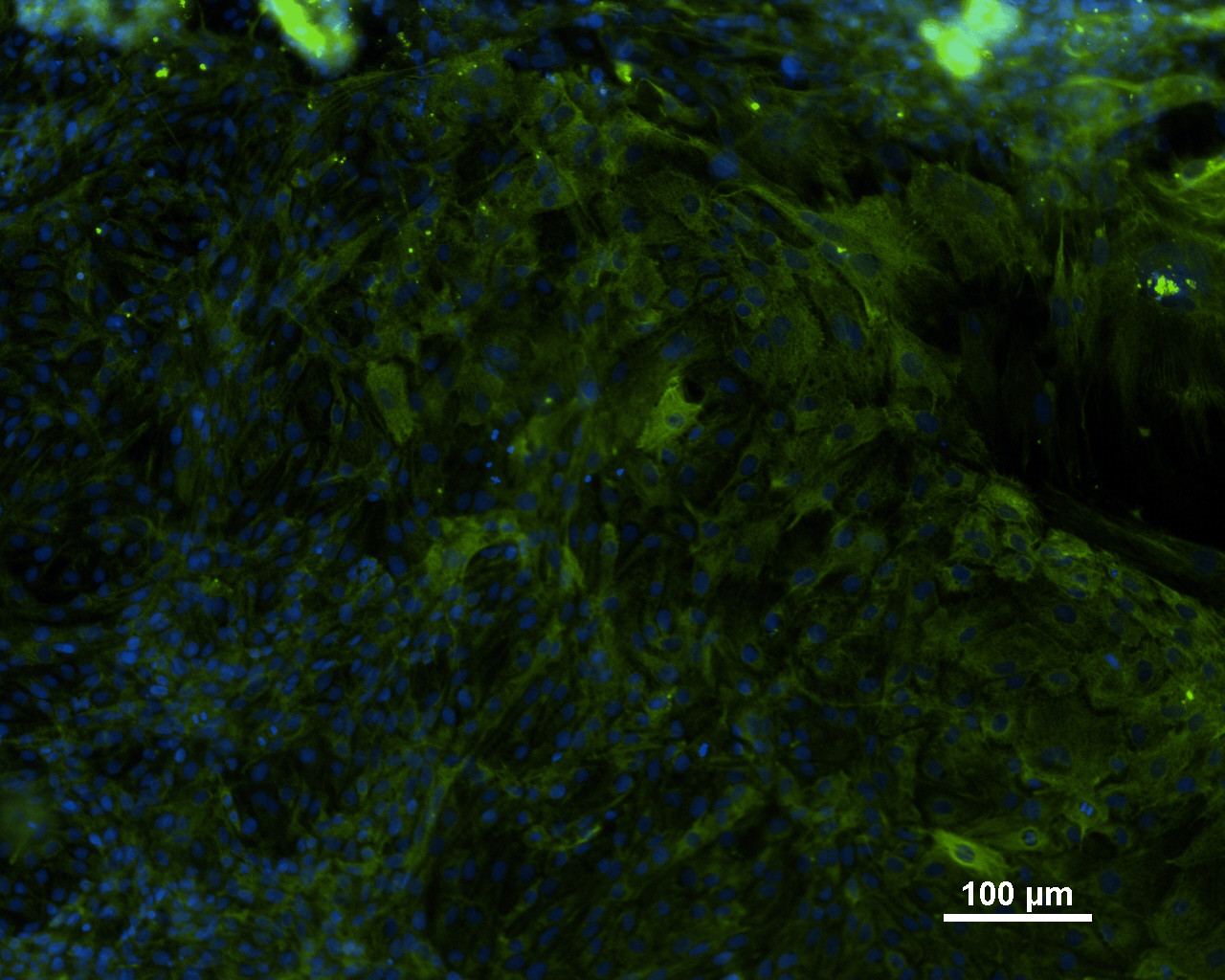
Analysis of Undifferentiated Cells
| Marker | Expressed | Immunostaining | RT-PCR | Flow Cytometry | Enzymatic Assay | Expression Profiles |
| POU5F1 (OCT-4) |
Yes |
|||||
| SSEA-4 |
Yes |
|||||
| SOX2 |
Yes |
|||||
| NANOG |
Yes |
|||||
| TRA 1-60 |
Yes |
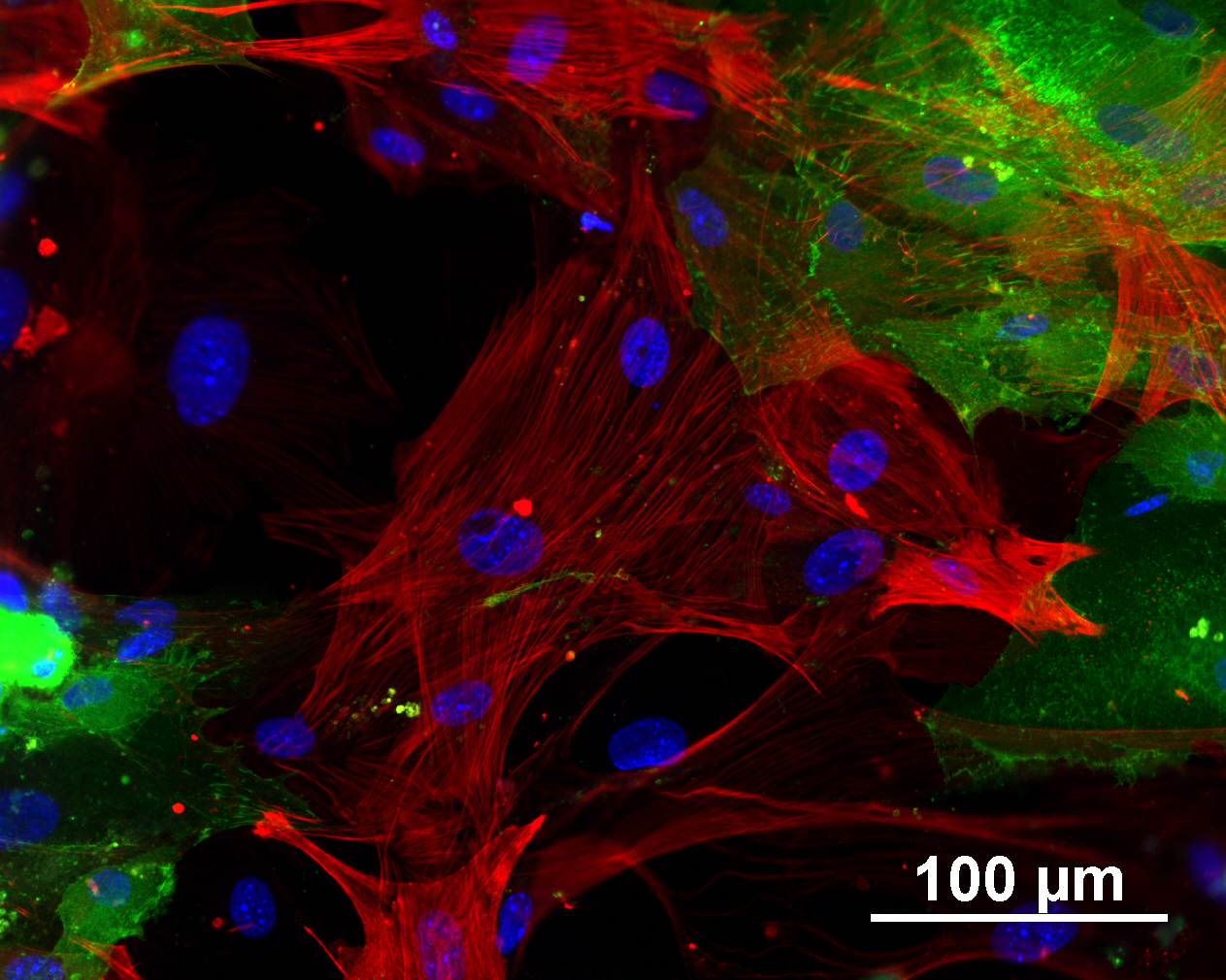
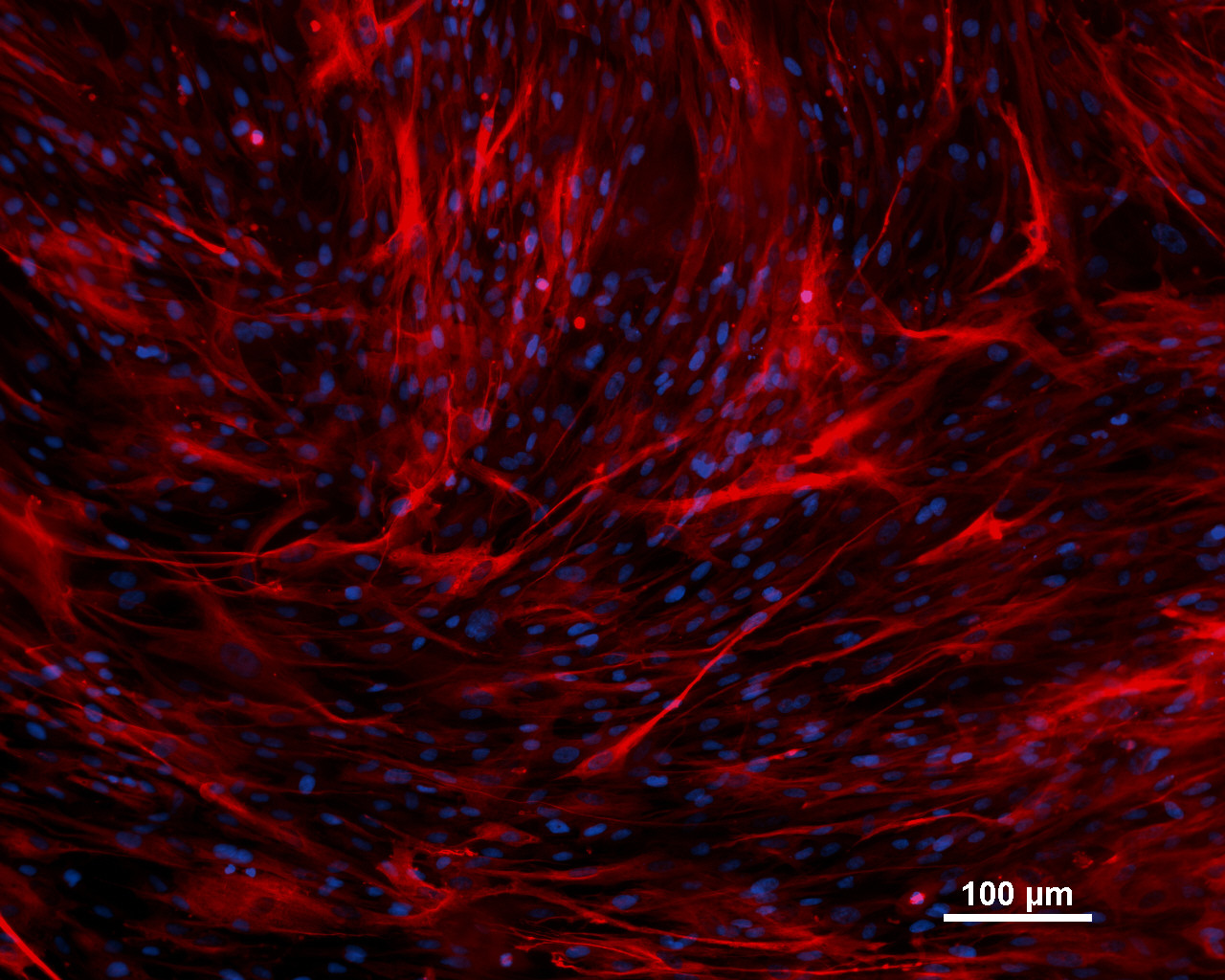
Differentiation Potency
In vitro spontaneous differentiation
| Marker | Expressed |
| CD29 |
Yes |
| Actin, alpha 2, smooth muscle, aorta |
Yes |
Microbiology / Virus Screening |
|
| Mycoplasma | Negative |
Genotyping
Karyotyping (Cell Line) |
|
| Has the cell line karyotype been analysed? |
Yes
Karyotyping and G-banding show ICGi052-B-1 iPSCs have a normal 46,XX karyotype at passage 10.
Passage number: 10
Karyotyping method:
G-Banding
|
Other Genotyping (Cell Line) |
|
Genetic Modification
| Genetic modifications not related to a disease |
|


Login to share your feedback, experiences or results with the research community.